Pathogenic role of acyl coenzyme A binding protein (ACBP) in Cushing's syndrome
- PMID: 39578649
- PMCID: PMC11659162
- DOI: 10.1038/s42255-024-01170-0
Pathogenic role of acyl coenzyme A binding protein (ACBP) in Cushing's syndrome
Abstract
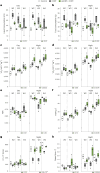
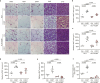
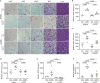
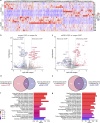
Cushing's syndrome is caused by an elevation of endogenous or pharmacologically administered glucocorticoids. Acyl coenzyme A binding protein (ACBP, encoded by the gene diazepam binding inhibitor, Dbi) stimulates food intake and lipo-anabolic reactions. Here we found that plasma ACBP/DBI concentrations were elevated in patients and mice with Cushing's syndrome. We used several methods for ACBP/DBI inhibition in mice, namely, (1) induction of ACBP/DBI autoantibodies, (2) injection of a neutralizing monoclonal antibody, (3) body-wide or hepatocyte-specific knockout of the Dbi gene, (4) mutation of the ACBP/DBI receptor Gabrg2 and (5) injections of triiodothyronine or (6) the thyroid hormone receptor-β agonist resmetirom to block Dbi transcription. These six approaches abolished manifestations of Cushing's syndrome such as increased food intake, weight gain, excessive adiposity, liver damage, hypertriglyceridaemia and type 2 diabetes. In conclusion, it appears that ACBP/DBI constitutes an actionable target that is causally involved in the development of Cushing's syndrome.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: O.K. is a scientific co-founder of Samsara Therapeutics. I.M. consults for Osasuna Therapeutics. G.K. has been holding research contracts with Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, PharmaMar, Osasuna Therapeutics, Samsara Therapeutics, Sanofi, Sutro, Tollys and Vascage. G.K. is on the Board of Directors of the Bristol Myers Squibb Foundation France. G.K. is a scientific co-founder of everImmune, Osasuna Therapeutics, Samsara Therapeutics and Therafast Bio; is in the scientific advisory boards of Hevolution, Institut Servier, Longevity Vision Funds and Rejuveron Life Sciences; and is the inventor of patents covering therapeutic targeting of ageing, cancer, cystic fibrosis and metabolic disorders. Among these patents, one, ‘Methods for weight reduction’ (US11905330B1), is relevant to this study. G.K.’s brother, Romano Kroemer, was an employee of Sanofi and now consults for Boehringer-Ingelheim. G.K.’s wife, Laurence Zitvogel, has held research contracts with GSK, Incyte, Lytix, Kaleido, Innovate Pharma, Daiichi Sankyo, Pilege, Merus, Transgene, 9m, Tusk and Roche, was on the on the Board of Directors of Transgene, is a co-founder of everImmune and holds patents covering the treatment of cancer and the therapeutic manipulation of the microbiota. The funders had no role in the design of the study, in the writing of the manuscript or in the decision to publish the results. The other authors declare no competing interests.
Figures












References
-
- Lacroix, A., Feelders, R. A., Stratakis, C. A. & Nieman, L. K. Cushing’s syndrome. Lancet386, 913–927 (2015). - PubMed
-
- Loriaux, D. L. Diagnosis and differential diagnosis of Cushing’s syndrome. N. Engl. J. Med.376, 1451–1459 (2017). - PubMed
-
- Pivonello, R. et al. Complications of Cushing’s syndrome: state of the art. Lancet Diabetes Endocrinol.4, 611–629 (2016). - PubMed
-
- Hasenmajer, V. et al. The immune system in Cushing’s syndrome. Trends Endocrinol. Metab.31, 655–669 (2020). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

