Efficacy of amniotic fluid, blood and urine samples for the diagnosis of toxoplasmosis in pregnant women candidates for amniocentesis using serological and molecular techniques
- PMID: 39578753
- PMCID: PMC11583409
- DOI: 10.1186/s12884-024-06979-x
Efficacy of amniotic fluid, blood and urine samples for the diagnosis of toxoplasmosis in pregnant women candidates for amniocentesis using serological and molecular techniques
Abstract
Backgrounds: Toxoplasmosis, a prevalent parasitic infection, is primarily caused by Toxoplasma gondii (T. gondii). This infection poses a significant threat to neonates during pregnancy and individuals with compromised immune systems. Consequently, it is imperative to develop a novel diagnostic approach that combines high sensitivity with low-risk sampling to effectively manage patients. The aim of this study is to utilize serological and molecular techniques for the diagnosis of T. gondii infection in 100 pregnant women who were under the care of a gynecologist and were candidates for amniocentesis.
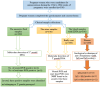
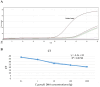
Methods: During the 15-19th weeks of pregnancy, a total of 100 samples each of amniotic fluid, buffy coat, plasma, and urine simultaneously were collected from pregnant women candidates for amniocentesis in Mazandaran province, northern Iran. This study involved various assessments: (1) detecting anti-T. gondii IgM and IgG in plasma through chemiluminescence assay (2) determining IgG avidity in plasma using the Enzyme-linked immunosorbent assay technique (3) identifying of T. gondii DNA in amniotic fluid, buffy coat and urine by nested PCR (nPCR) and quantitative real-time PCR (qPCR) methods targeting the REP-529 gene, as well as genotyping using GRA6 target genes, and (4) assessing the sensitivity and specificity of the nPCR and qPCR tests.
Results: Out of 100 pregnant women screened, 70 were between the ages of 31 to 40 years old. Among them, 23 and 44 had one and two previous pregnancies. Additionally, 13 and 8 women had one and two history of abortions, respectively. Following serologic testing, 52% of the individuals were positive for T. gondii antibodies. Of these, 52 samples were positive for IgG antibodies, and one sample was positive for both IgG and IgM antibodies. Notably, all 52 cases with IgG positivity exhibited a high level of IgG avidity. Regarding the molecular testing of amniotic fluid samples, two pregnant women tested positive in the nPCR assay, while three tested positive in the qPCR assay. Furthermore, genotyping revealed that all positive samples belonged to type I of the T. gondii genotype. Moreover, none of the 100 buffy coat and urine samples tested positive for T. gondii using the nPCR and qPCR techniques.
Conclusion: The findings of the current study suggest that serological methods alone may not be reliable in diagnosing congenital toxoplasmosis and cannot rule out the diagnosis of toxoplasmosis and must be approved by molecular tests.
Keywords: Toxoplasma Gondii; Amniocentesis; Nested PCR; Pregnant women; Real-time PCR.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was ethically approved by the Ethics Committee of Mazandaran University of Medical Sciences (IR.MAZUMS.REC.1400.11546), and all procedures adhered to ethical rules and principles set forth in the Declaration of Helsinki. Also, Informed consent was obtained from all the participants. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Rahimi Esboei B, Fallahi S, Zarei M, Kazemi B, Mohebali M, Shojaee S, et al. Utility of blood as the clinical specimen for the diagnosis of ocular toxoplasmosis using uracil DNA glycosylase-supplemented loop-mediated isothermal amplification and real-time polymerase chain reaction assays based on REP-529 sequence and B1 gene. BMC Infect Dis. 2022;22(1):1–10. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

