D-PRISM: a global survey-based study to assess diagnostic and treatment approaches in pneumonia managed in intensive care
- PMID: 39578900
- PMCID: PMC11585090
- DOI: 10.1186/s13054-024-05180-y
D-PRISM: a global survey-based study to assess diagnostic and treatment approaches in pneumonia managed in intensive care
Erratum in
-
Correction: D-PRISM: a global survey-based study to assess diagnostic and treatment approaches in pneumonia managed in intensive care.Crit Care. 2025 Oct 16;29(1):440. doi: 10.1186/s13054-025-05610-5. Crit Care. 2025. PMID: 41102822 Free PMC article. No abstract available.
Abstract
Background: Pneumonia remains a significant global health concern, particularly among those requiring admission to the intensive care unit (ICU). Despite the availability of international guidelines, there remains heterogeneity in clinical management. The D-PRISM study aimed to develop a global overview of how pneumonias (i.e., community-acquired (CAP), hospital-acquired (HAP), and Ventilator-associated pneumonia (VAP)) are diagnosed and treated in the ICU and compare differences in clinical practice worldwide.
Methods: The D-PRISM study was a multinational, survey-based investigation to assess the diagnosis and treatment of pneumonia in the ICU. A self-administered online questionnaire was distributed to intensive care clinicians from 72 countries between September to November 2022. The questionnaire included sections on professional profiles, current clinical practice in diagnosing and managing CAP, HAP, and VAP, and the availability of microbiology diagnostic tests. Multivariable analysis using multiple regression analysis was used to assess the relationship between reported antibiotic duration and organisational variables collected in the study.
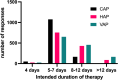
Results: A total of 1296 valid responses were collected from ICU clinicians, spread between low-and-middle income (LMIC) and high-income countries (HIC), with LMIC respondents comprising 51% of respondents. There is heterogeneity across the diagnostic processes, including clinical assessment, where 30% (389) did not consider radiological evidence essential to diagnose pneumonia, variable collection of microbiological samples, and use and practice in bronchoscopy. Microbiological diagnostics were least frequently available in low and lower-middle-income nation settings. Modal intended antibiotic treatment duration was 5-7 days for all types of pneumonia. Shorter durations of antibiotic treatment were associated with antimicrobial stewardship (AMS) programs, high national income status, and formal intensive care training.
Conclusions: This study highlighted variations in clinical practice and diagnostic capabilities for pneumonia, particularly issues with access to diagnostic tools in LMICs were identified. There is a clear need for improved adherence to existing guidelines and standardized approaches to diagnosing and treating pneumonia in the ICU. Trial registration As a survey of current practice, this study was not registered. It was reviewed and endorsed by the European Society of Intensive Care Medicine.
Keywords: Antimicrobials; Bronchoscopy; Community-acquired; Hospital-acquired; Intensive care unit (ICU); Pneumonia; Surveys and questionnaires; Ventilator-associated.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This multinational cross-sectional study conducted an online self-administered questionnaire to intensive care clinicians. As an anonymized survey of clinical practice without individual patient data, the requirement for research ethics approval and formal written consent was waived by the UK Health Research Authority. Consent for publication: Not applicable. Competing interests: Nathan D. Nielsen sits on the scientific advisory boards of Adrenomed AG and Inotrem. Jordi Rello received honoraria from the Speakers' Bureau and consultancies from ROCHE, Pfizer & MSD. Andrew Conway Morris reports speaking fees from Biomerieux, Thermo-Fisher, Fischer and Paykel and Boston Scientific, he sits on the scientific advisory board of Cambridge Infection Diagnostics. Pedro Póvoa reports honoraria for lectures and advisory boards from Merck Sharp & Dohme, Gilead, Mundipharma and Pfizer, and advisory boards from Merck Sharp & Dohme, Sanofi, Gilead and Biocodex. All other authors report no competing interests.
Figures
References
-
- Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociacion Latinoamericana del Torax (ALAT). Eur Respir J. 2017;50(3). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MED-357-2023/Universidad de La Sabana
- 2022YFC2504503/National Key Research and Development Program of China
- 82272180/National Natural Science Foundation of China
- CPA-Z06-ZC-2021-004/Project of Drug Clinical Evaluate Research of Chinese Pharmaceutical Association
- P400PM_183865/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous