Trial protocol for SiroSkin: a randomised double-blind placebo-controlled trial of topical sirolimus in chemoprevention of facial squamous cell carcinomas in solid organ transplant recipients
- PMID: 39578921
- PMCID: PMC11585096
- DOI: 10.1186/s13063-024-08619-3
Trial protocol for SiroSkin: a randomised double-blind placebo-controlled trial of topical sirolimus in chemoprevention of facial squamous cell carcinomas in solid organ transplant recipients
Abstract
Background: Keratinocyte carcinomas such as basal cell carcinomas and squamous cell carcinomas are a major burden affecting morbidity and mortality in solid organ transplant recipients (SOTRs). Best treatment includes frequent skin checks for early detection and surgery for high incidence of skin cancers. Sirolimus is an immunosuppressive drug which may reduce the burden of skin cancer but may be poorly tolerated when given orally. Topical sirolimus has been proven effective at reducing the burden of skin cancers in animal models, and its safety has long been established in children with tuberous sclerosis. A recent 12-week phase II trial of topical sirolimus suggested it was safe and effective at reducing the early signs of skin cancer in the absence of major side effects. The aim of the SiroSkin trial is to determine whether topical sirolimus can fill a major gap in current therapies by reducing the onset and number of new skin cancers thus reducing burden of disease and cost-effectiveness.
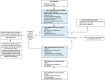
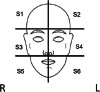
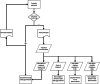
Methods: Protocol for a multi-centred phase III, participant- and clinician assessor-blinded, placebo-controlled randomised trial in SOTRs. A minimum 146 participants randomised 1:1 will be treated with 1% topical sirolimus versus placebo applied to the face on a regular basis for 24 weeks. Participation is 24 months in total-24 weeks of treatment and 18 months of follow-up. Outcomes include the number of keratinocyte carcinomas at 24 weeks of treatment compared to placebo and then at 12 and 24 months after initiation of treatment. Analysis will be as per protocol and intention to treat.
Discussion: The results of this trial will inform management strategies for skin cancers in SOTRs and provide evidence for cost-effectiveness.
Trial registration: Clinicaltrials.gov NCT05860881. Registered on June 15, 2023, and on anzctr.org.au (registration number NCT05860881).
Keywords: Actinic keratosis; Basal cell carcinoma; Sirolimus; Skin cancer; Solid organ transplant recipients; Squamous cell carcinoma; Topical 5-fluorouracile; Topical sirolimus.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate {24}: The study was approved by Metro South Human Research Ethics Committee on 18 April 2023 (HREC/2023/QMS/95325). All participants will have to provide written informed consent before participation. Consent for publication {32}: This is not applicable. No identifying images or other personal or clinical details of participants are presented here or will be presented in reports of the trial results. The participant information materials and informed consent form are available from the corresponding author on request. All authors listed above meet the authorship criteria according to the latest guidelines of the International Committee of Medical Journal Editors, and all authors agree with the manuscript. Competing interests {28}: The authors declare that they have no competing interests.
Figures
References
-
- Moloney FJ, Comber H, O’Lorcain P, et al. A population-based study of skin cancer incidence and prevalence in renal transplant recipients. Br J Dermatol. 2006;154(3):498–504. - PubMed
-
- Bouwes Bavinck JN, Hardie DR, Green A, et al. The risk of skin cancer in renal transplant recipients in Queensland, Australia. A follow-up study. Transplantation. 1996;61(5):715–21. - PubMed
-
- Iannacone MR, Sinnya S, Pandeya N, et al. Prevalence of skin cancer and related skin tumors in high-risk kidney and liver transplant recipients in Queensland, Australia. J Invest Dermatol. 2016;136(7):1382–6. - PubMed
-
- Ong CS, Keogh AM, Kossard S, et al. Skin cancer in Australian heart transplant recipients. J Am Acad Dermatol. 1999;40(1):27–34. - PubMed
-
- Na R, Grulich AE, Meagher NS, et al. De novo cancer-related death in Australian liver and cardiothoracic transplant recipients. Am J Transplant. 2013;13(5):1296–304. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous