Consensus Statement: Recommendations on Actionable Biomarker Testing for Thyroid Cancer Management
- PMID: 39579327
- PMCID: PMC11659332
- DOI: 10.1007/s12022-024-09836-x
Consensus Statement: Recommendations on Actionable Biomarker Testing for Thyroid Cancer Management
Erratum in
-
Correction: Consensus Statement: Recommendations on Actionable Biomarker Testing for Thyroid Cancer Management.Endocr Pathol. 2024 Dec;35(4):309-310. doi: 10.1007/s12022-024-09843-y. Endocr Pathol. 2024. PMID: 39641850 Free PMC article. No abstract available.
Abstract
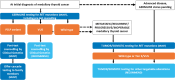
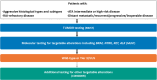
Thyroid cancer management is rapidly changing. The identification of actionable biomarkers through both germline and somatic testing are now an integral part of directing patient management. However, deficiencies and disparities within existing thyroid cancer biomarker test approaches are resulting in inconsistent application for patient care. An expert panel was convened to create consensus biomarker testing algorithms and recommendations on actionable biomarker testing for patients diagnosed with medullary thyroid cancer, non-anaplastic follicular cell-derived thyroid cancer, or anaplastic follicular cell-derived thyroid cancer who may benefit from targeted therapies. A review of international guidelines was performed to determine the current state, and a literature review was carried out to further evaluate the evidence supporting the use of actionable biomarkers in patients diagnosed with thyroid cancer. Thyroid biomarker-related gaps impacting patient care were also discussed, with an emphasis on the importance of a multidisciplinary team approach for optimal patient care. The recommendations are presented with the aim to help physicians navigate the current thyroid cancer biomarker testing landscape with its many challenges, balancing aspirational care with what is practical and feasible in terms of economic realities and jurisdictional constraints. By remaining therapy-agnostic, these algorithms and recommendations are broadly applicable.
Keywords: ALK; BRAF; NTRK; RAS; RET; Algorithms; Biomarkers; Cancer management; Familial thyroid carcinoma; Genetic testing; IHC; Immunohistochemistry; Medullary thyroid carcinoma; Microsatellite instability; Mismatch repair; Molecular testing; NGS; Next-generation sequencing; PD-L1; Predictive; Targeted therapy; Thyroid cancer; Tumor mutational burden.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics Approval: Not applicable for this publication. Consent for Publication: All authors approved the final version and publication of the manuscript. Competing Interests: All authors report honoraria (facilitated via Precision RxDx) from Eli Lilly Canada for participation in working group meetings related to this project. Dr. Ozgur Mete is the Editor-in-Chief of Endocrine Pathology. This article is handled by an independent senior editor as per Springer Nature’s policies. Dr. Mete received honorarium for serving as an advisory board member for Bayer. Dr. Andrée Boucher has received research support from Eisai and has been compensated for being a speaker and serving as a board member or on an advisory panel for Bayer and Eisai. Dr. Omar Abdel-Rahman has received consulting fees from Amgen, Eisai, Ipsen, Lilly, and Roche, honoraria from Bayer. Dr. Houda Bahig has received grants from AstraZeneca, Sanofi and Varian Medical Systems, as well as consulting fees from AstraZeneca and EMD Serono. Dr. Cheryl Ho received grants from AstraZeneca and Roche which were paid to her institution. She received honoraria for advisory board attendance from AbbVie, Amgen, AstraZeneca, Bayer, BMS, Janssen, Jazz, Merck, Novartis, Pfizer, Roche, and Sanofi. Dr. Kasmintan A. Schrader has a consulting and advisory relationship with and has received honoraria from AstraZeneca Canada and Pfizer and research funding from AstraZeneca Canada and Merck. Dr. Eric Winquist received grants from Eli Lilly, Merck, and Novartis which were paid to his institution. He received consulting fees for advisory boards from Bayer, BMS, Eisai, EMD Serono, Merck, and Roche; honoraria from EMD Serono and support for attending meetings from Eisai. Dr. Jonn Wu served as an Executive board member, International Society of Oral Oncology, from 2019 to 2023. Dr. Shereen Ezzat has served as advisory board member/consultant for Bayer, Eisai, Eli Lilly, Ipsen, Novartis, Medunik Canada, Merck, Pfizer, and Recordati Rare Diseases. Dr. Nicole Chau has received research funding from BeiGene, BMS, ERASCA, Merck, and Roche which were paid to her institution. She received consulting fees for advisory boards from Bayer, BMS, Eisai, Eli Lilly, EMD Serono, Ipsen, Merck, Novartis, Pfizer, and Roche. Dr. Olfat Kamel Hasan, Dr. Bernard Lemieux, and Dr. Ralph Wong have no conflicts of interest to declare that are relevant to the content of this article.
Figures


References
-
- WHO Classification of Tumours Editorial Board: Endocrine and Neuroendocrine tumours, vol. 8. 5th edn. (International Agency for Research on Cancer, Lyon, France, 2022) https://tumourclassification.iarc.who.int, 2022.
-
- Christofer Juhlin C, Mete O, Baloch ZW. The 2022 WHO classification of thyroid tumors: novel concepts in nomenclature and grading. Endocrine-related cancer 30(2):e220293, 2023. - PubMed
-
- Vissio E, Maletta F, Fissore J et al. External Validation of Three Available Grading Systems for Medullary Thyroid Carcinoma in a Single Institution Cohort. Endocr Pathol 33: 359-370, 2022. - PubMed
-
- Williams JF, Zhao M, Najdawi F et al. Grading of Medullary Thyroid Carcinoma: an Interobserver Reproducibility Study. Endocr Pathol 33: 371-377, 2022. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

