Influence of ethnicity on cyclin-dependent kinase inhibitor efficacy and toxicity: A systematic review and meta-analysis
- PMID: 39579620
- PMCID: PMC11616569
- DOI: 10.1016/j.breast.2024.103833
Influence of ethnicity on cyclin-dependent kinase inhibitor efficacy and toxicity: A systematic review and meta-analysis
Abstract
Background: The combination of cyclin-dependent kinase 4 and 6 inhibitors (CDK4/6i) with endocrine therapy (ET) is the standard of care for patients with hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR+/HER2-) advanced breast cancer (aBC). While the efficacy and safety profiles of CDK4/6i and ET have been extensively evaluated in phase II and III trials worldwide, it remains unclear whether the response to CDK4/6i and toxicity profile vary among Asian and non-Asian patients. Therefore, we aimed to assess the treatment efficacy of ET with and without CDK4/6i by comparing outcomes in Asian and non-Asian subgroups included in these clinical trials. In addition, we evaluated the toxicity profiles of the treatments by estimating the risk of treatment-related adverse events (AEs).
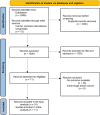
Methods: We conducted a meta-analysis including the most recent randomized trial data systematically searched from PubMed, Embase, Web of Science, Cochrane CENTRAL (from inception to May 31st, 2024) or presented in abstracts or oral presentations at the ESMO, ASCO, and SABCS international congresses. We included studies comparing CDK4/6i (palbociclib, ribociclib, abemaciclib, dalpiciclib) + ET versus placebo + ET. Progression-free survival (PFS) and overall survival (OS), hazard ratios (HR), and 95 % confidence intervals (CI) were extracted for the two subgroups of interest. To evaluate the treatment-related toxicity profiles, we extracted the number of side effects to estimate the risk of treatment-emergent AEs.
Results: Eleven studies (n = 5129) were included in this meta-analysis. The addition of CDK4/6i to ET consistently improved PFS in both Asian (HR = 0.52, 95 % CI 0.47-0.60; p < 0.001) and non-Asian (HR = 0.58, 95 % CI 0.52-0.64; p < 0.001) groups. Similarly, the combination of CDK4/6i + ET led to an OS improvement in both Asian (HR = 0.75, 95 % CI 0.62-0.91; p = 0.003) and non-Asian (HR = 0.81, 95 % CI 0.73-0.89; p < 0.001) patients. The risk of treatment related toxicity was higher in the CDK4/6i + ET arm in both Asian and non-Asian groups. Interestingly, a numerically higher rate of treatment-related hematological toxicity was observed in Asian patients, although no significant interethnic difference was found in the relative risk of these events.
Conclusions: The combination of CDK4/6i and ET significantly improves PFS and OS compared to ET alone in both Asian and non-Asian patients with HR+/HER2-aBC. Although the magnitude of benefit appears to be independent of ethnicity, future clinical trials should devise a standardized method for stratifying patients by ethnicity to more effectively assess potential differences in treatment benefits.
Systematic review registration: PROSPERO registration number: CRD42024543217.
Keywords: Asian; Breast cancer; CDK4/6i; Ethnicity; Meta-analysis; Toxicity.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Martina Pagliuca reports a relationship with Gilead that includes: funding grants. Michelino De Laurentiis reports a relationship with Roche, Novartis, Lilly, Pierre Fabre, AstraZeneca, MSD, Seagen, Gilead, Daiichi Sankyo, Pfizer and Exact science that includes: consulting or advisory. Michelino De Laurentiis reports a relationship with Roche, Novartis, Lilly, Pierre Fabre, AstraZeneca,MSD, Daiichi Sankyo, Exact science, Gilead, Ipsen, Pfizer, Seagen, Takeda, Sanofi-Genzyme that includes: funding grants. Fabio Puglisi reports a relationship with mgen, AstraZeneca, Daichii Sankyo, Celgene, Eisai, Lilly, Gilead, Ipsen, MSD, Novartis, Pierre Fabre, Pfizer, Roche, Seagen, Takeda and Viatris that includes: consulting or advisory, funding grants, and travel reimbursement. Lucia Del Mastro reports a relationship with Lilly, Novartis, Roche, Pfizer, Daiichi Sankyo, Exact science, Gilead, Pierre Fabre, Eisai, AstraZeneca and Agendia that includes: consulting or advisory. Lucia Del Mastro reports a relationship with Roche, Pfizer, Lilly, MSD, Seagen, Gilead, Pierre Fabre, Eisai, Ipsen, Exact science, AstraZeneca and Novartis that includes: funding grants. Mario Giuliano reports a relationship with AstraZeneca, Daichii Sankyo, Exact Sciences, Lilly, MSD, Novartis, Pfizer, Roche, Seagen that includes: consulting or advisory. Mario Giuliano reports a relationship with Roche, Celgene, Pfizer that includes: travel reimbursement. Carmine De Angelis reports a relationship with Novartis, GSK, Eli Lilly, and Pfizer. that includes: consulting or advisory. Grazia Arpino reports a relationship with Roche, Pfizer, Lilly, MSD, AstraZeneca, Novartis that includes: consulting or advisory. Grazia Arpino reports a relationship with Roche, Pfizer, Lilly, MSD, AstraZeneca and Novartis that includes: consulting or advisory and funding grants. Roberta Caputo reports a relationship with Novartis, Lilly, Astra Zeneca, Daichii Sankyo, Veracyte, Pfizer that includes: funding grants. Roberta Caputo reports a relationship with Roche, Astra Zeneca, Lilly, Daichii Sankyo, Novartis, Seagen, MSD, Gilead, that includes: consulting or advisory. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







Similar articles
-
Abemaciclib Plus Fulvestrant in Advanced Breast Cancer After Progression on CDK4/6 Inhibition: Results From the Phase III postMONARCH Trial.J Clin Oncol. 2025 Mar 20;43(9):1101-1112. doi: 10.1200/JCO-24-02086. Epub 2024 Dec 18. J Clin Oncol. 2025. PMID: 39693591 Free PMC article. Clinical Trial.
-
Efficacy of Subsequent Treatments After Disease Progression on CDK4/6 Inhibitors in Patients With Hormone Receptor-Positive Advanced Breast Cancer.JCO Oncol Pract. 2025 Jun;21(6):832-842. doi: 10.1200/OP-24-00649. Epub 2024 Dec 17. JCO Oncol Pract. 2025. PMID: 39689274
-
Safety and quality of life of CDK4/6 inhibitors therapy for hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: a multicenter cross-sectional survey in China.BMC Cancer. 2025 May 27;25(1):951. doi: 10.1186/s12885-025-14223-8. BMC Cancer. 2025. PMID: 40426093 Free PMC article.
-
Endocrine therapy-based treatments in hormone receptor-positive/HER2-negative advanced breast cancer: systematic review and network meta-analysis.ESMO Open. 2020 Aug;5(4):e000842. doi: 10.1136/esmoopen-2020-000842. ESMO Open. 2020. PMID: 32847835 Free PMC article.
-
Survival Following CDK4/6 Inhibitor Therapy for Hormone Receptor-Positive, ERBB2-Negative Metastatic Breast Cancer.JAMA Netw Open. 2025 Feb 3;8(2):e2461067. doi: 10.1001/jamanetworkopen.2024.61067. JAMA Netw Open. 2025. PMID: 39982725 Free PMC article.
References
-
- Slamon D.J., Neven P., Chia S., Fasching P.A., De Laurentiis M., Im S.-A., et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36:2465–2472. doi: 10.1200/JCO.2018.78.9909. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

