Abdominal ultrasound stimulation alleviates DSS-induced colitis and behavioral disorders in mice by mediating the microbiota-gut-brain axis balance
- PMID: 39580323
- PMCID: PMC12014354
- DOI: 10.1016/j.neurot.2024.e00494
Abdominal ultrasound stimulation alleviates DSS-induced colitis and behavioral disorders in mice by mediating the microbiota-gut-brain axis balance
Abstract
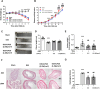
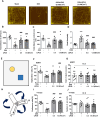
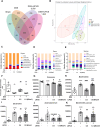
Inflammatory bowel disease (IBD) has the potential to induce neuroinflammation, which may increase the risk of developing neurodegenerative disorders. Ultrasound stimulation to the abdomen is a potential treatment for dextran sulfate sodium (DSS)-induced acute colitis. The present study aimed to investigate whether abdominal low-intensity pulsed ultrasound (LIPUS) can alleviate DSS-induced neuroinflammation through the microbiota-gut-brain axis. Male mice were fed DSS to induce ulcerative colitis. LIPUS stimulation was then applied to the abdomen at intensities of 0.5 and 1.0 W/cm2. Mouse biological samples were analyzed, and behavior was evaluated. [18F]FEPPA PET/CT imaging was employed to track and quantify inflammation in the abdomen and brain. Changes in the gut microbiota composition were analyzed using 16S rRNA sequencing. Abdominal LIPUS significantly inhibited the DSS-induced inflammatory response, repaired destroyed crypts, and partially preserved the epithelial barrier. [18F]FEPPA accumulation in the colitis-induced neuroinflammation in the abdomen and specific brain regions significantly decreased after LIPUS treatment. LIPUS maintained intestinal integrity by increasing zonula occludens and occludin levels, reduced lipopolysaccharide-binding protein and lipopolysaccharide levels in the serum, and improved behavioral dysfunctions. Moreover, LIPUS, at an intensity of 0.5 W/cm2, reshaped the gut microbiota in colitis-induced mice by increasing the relative abundance of the Firmicutes and decreasing the relative abundance of the Bacteroidota. Our findings demonstrated that abdominal LIPUS stimulation has the potential to be a novel therapeutic strategy to improve colitis-induced behavioral disorders through microbiota-gut-brain axis signaling.
Keywords: Abdominal ultrasound; IBD; Intestinal inflammation; Microbiota; Neuroinflammation.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Ng S.C., Shi H.Y., Hamidi N., Underwood F.E., Tang W., Benchimol E.I., et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390(10114):2769–2778. - PubMed
-
- He R., Li Y., Han C., Lin R., Qian W., Hou X. L-Fucose ameliorates DSS-induced acute colitis via inhibiting macrophage M1 polarization and inhibiting NLRP3 inflammasome and NF-kB activation. Int Immunopharm. 2019;73:379–388. - PubMed
-
- Zhao B., Wu J., Li J., Bai Y., Luo Y., Ji B., et al. Lycopene alleviates DSS-induced colitis and behavioral disorders via mediating microbes-gut-brain Axis balance. J Agric Food Chem. 2020;68(13):3963–3975. - PubMed
-
- Horst S., Chao A., Rosen M., Nohl A., Duley C., Wagnon J.H., et al. Treatment with immunosuppressive therapy may improve depressive symptoms in patients with inflammatory bowel disease. Dig Dis Sci. 2015;60(2):465–470. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

