Enhancing antitumor immunity in Lewis lung cancer through plasma-treated medium-induced activation of dendritic cells
- PMID: 39580412
- PMCID: PMC11585098
- DOI: 10.1186/s12935-024-03569-x
Enhancing antitumor immunity in Lewis lung cancer through plasma-treated medium-induced activation of dendritic cells
Abstract
Background: Recently, atmospheric non-thermal plasma jet-treated medium (PTM) has been recognized as a novel strategy in cancer therapy and lymphocyte activation. However, PTM has limitations in inducing a robust antitumor-immune response. This study demonstrated that PTM treatment inhibited tumor progression by activating dendritic cells (DCs).
Method: In this study, we investigated the effects of PTM on selective cytotoxicity and intracellular reactive oxygen species (ROS) generation and oxidative stress-mediated signaling (e.g., glutathione peroxidase, catalase) using respective fluorescence probes in Lewis lung cancer (LLC) cells. Then, the PTM affects the expression of interferon-gamma (IFN)-γ-induced programmed death-ligand 1 (PD-L1) and inhibition of signal transducer and activator of transcription 1 (STAT1) in LLC cells using immunoblotting. Additionally, PTM effects on the tumor cell's death and activation of DCs were done by co-culturing DCs with or without tumor cells. Further, a mouse model was used to evaluate the synergistic antitumor effects of PTM and DCs where tumors are grown under the skin.
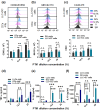
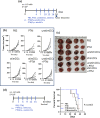
Results: PTM-exposed tumor cells increase intracellular superoxide production, enhancing ROS generation and leading to cancer immunogenic cell death. In addition, PTM suppresses IFN-γ-induced PD-L1 expression and STAT1 activation in tumor cells. The activation of DCs induced by PTM is downregulated when these cells are co-cultured with tumor cells. In vivo, intraperitoneal injection of PTM-activated DCs, as a synergistic agent to intertumoral PTM treatment, led to increased CD4+ and CD8+ T cell infiltration into the tumor and spleen and eventually decreased tumor growth.
Conclusion: Overall, this research introduces a promising avenue for improving lung cancer treatment using PTM to stimulate an immune response and induce cell death in tumor cells. Further studies will be essential to validate these findings and explore clinical applications.
Keywords: Cancer immunogenic cell death; Dendritic cell activation; Lewis Lung cancer cells; Non-thermal plasma-treated medium; Programmed death-ligand 1; Reactive oxygen species.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The Institutional Research and Ethics Committee of Chungnam National University approved all animal experiments (approval number: 202003 A-CNU-064), which complied with the relevant guidelines of the Korean Food and Drug Administration. Competing interests: The authors declare no competing interests.
Figures





Similar articles
-
In situ delivery of iPSC-derived dendritic cells with local radiotherapy generates systemic antitumor immunity and potentiates PD-L1 blockade in preclinical poorly immunogenic tumor models.J Immunother Cancer. 2021 May;9(5):e002432. doi: 10.1136/jitc-2021-002432. J Immunother Cancer. 2021. PMID: 34049930 Free PMC article.
-
FOXA1 enhances antitumor immunity via repressing interferon-induced PD-L1 expression in nasopharyngeal carcinoma.J Immunother Cancer. 2024 Nov 14;12(11):e010091. doi: 10.1136/jitc-2024-010091. J Immunother Cancer. 2024. PMID: 39542656 Free PMC article.
-
Programmed death ligand 1 (PD-L1) plays a vital part in DC tolerogenicity induced by IFN-γ.Int Immunopharmacol. 2021 Oct;99:107978. doi: 10.1016/j.intimp.2021.107978. Epub 2021 Jul 20. Int Immunopharmacol. 2021. PMID: 34298399
-
Emerging role of natural products in cancer immunotherapy.Acta Pharm Sin B. 2022 Mar;12(3):1163-1185. doi: 10.1016/j.apsb.2021.08.020. Epub 2021 Aug 21. Acta Pharm Sin B. 2022. PMID: 35530162 Free PMC article. Review.
-
CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A review.J Cell Physiol. 2019 Jun;234(6):8509-8521. doi: 10.1002/jcp.27782. Epub 2018 Nov 22. J Cell Physiol. 2019. PMID: 30520029 Review.
Cited by
-
Immunological Control of Herpes Simplex Virus Type 1 Infection: A Non-Thermal Plasma-Based Approach.Viruses. 2025 Apr 23;17(5):600. doi: 10.3390/v17050600. Viruses. 2025. PMID: 40431612 Free PMC article. Review.
-
Chemical Composition and Anti-Lung Cancer Activities of Melaleuca quinquenervia Leaf Essential Oil: Integrating Gas Chromatography-Mass Spectrometry (GC/MS) Profiling, Network Pharmacology, and Molecular Docking.Pharmaceuticals (Basel). 2025 May 22;18(6):771. doi: 10.3390/ph18060771. Pharmaceuticals (Basel). 2025. PMID: 40573169 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

