The role of the estimand framework in the analysis of patient-reported outcomes in single-arm trials: a case study in oncology
- PMID: 39580440
- PMCID: PMC11585159
- DOI: 10.1186/s12874-024-02408-x
The role of the estimand framework in the analysis of patient-reported outcomes in single-arm trials: a case study in oncology
Abstract
Background: Patient-reported outcomes (PROs) play an increasing role in the evaluation of oncology treatments. At the same time, single-arm trials are commonly included in regulatory approval submissions. Because of the high risk of biases, results from single-arm trials require careful interpretation. This benefits from a clearly defined estimand, or target of estimation. In this case study, we demonstrated how the ICH E9 (R1) estimand framework can be implemented in SATs with PRO endpoints.
Methods: For the global quality of life outcome in a real single-arm lung cancer trial, a range of possible estimands was defined. We focused on the choice of the variable of interest and strategies to deal with intercurrent events (death, treatment discontinuation and disease progression). Statistical methods were described for each estimand and the corresponding results on the trial data were shown.
Results: Each intercurrent event handling strategy resulted in its own estimated mean global quality of life over time, with a specific interpretation, suitable for a corresponding clinical research aim. In the setting of this case study, a 'while alive' strategy for death and a 'treatment policy' strategy for non-terminal intercurrent events were deemed aligned with a descriptive research aim to inform clinicians and patients about expected quality of life after the start of treatment.
Conclusions: The results show that decisions made in the estimand framework are not trivial. Trial results and their interpretation strongly depend on the chosen estimand. The estimand framework provides a structure to match a research question with a clear target of estimation, supporting specific clinical decisions. Adherence to this framework can help improve the quality of data collection, analysis and reporting of PROs in SATs, impacting decision making in clinical practice.
Keywords: Estimand; Oncology; Patient-reported outcomes; Quality of life; Repeated measurements; Single-arm trial.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: No data were collected for the purpose of this study. Anonymized data from a previously published trial were shared with us for the illustration of analysis methods, respecting the informed consent previously provided by the trial participants. Consent for publication: Not applicable. Competing interests: Satrajit Roychoudhury, is an employee of Pfizer Inc., and a stockholder of Pfizer Inc. and Novartis Pharmaceutical. Els Goetghebeur and Dries Reynders contribute to a university-based statistical consulting service that has received limited consulting fees from Pfizer, no personal fees. There are no other potential conflicts of interests among the authors.
Figures

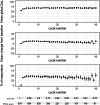
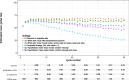


References
-
- Bottomley A, Pe M, Sloan J, Basch E, Bonnetain F, Calvert M, et al. Moving forward toward standardizing analysis of quality of life data in randomized cancer clinical trials. Clin Trials Lond Engl. 2018;15(6):624–30. - PubMed
-
- U.S. Department of Health and Human Services Food and Drug Administration. Guidance for Industry - Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. 2009. Available from: https://www.fda.gov/media/77832/download. Cited 2023 Aug 16.
-
- U.S. Department of Health and Human Services Food and Drug Administration. Guidance for Industry - Core Patient-Reported Outcomes in Cancer Clinical Trials (Draft Guidance). 2021. Available from: https://www.fda.gov/media/149994/download. Cited 2023 Aug 16.
-
- European Medicines Agency. Appendix 2 to the guideline on the evaluation of anticancer medicinal products in man. The use of patient-reported outcome (PRO) measures in oncology studies. European Medicines Agency; 2016. Available from: https://www.ema.europa.eu/en/documents/other/appendix-2-guideline-evalua.... Cited 2023 Apr 28.
-
- U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Patient-Focused Drug Development: Collecting Comprehensive and Representative Input - Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders (Final Guidance). 2020. Available from: https://www.fda.gov/media/139088/download. Cited 2023 Oct 18.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

