Crystal structures of DCAF1-PROTAC-WDR5 ternary complexes provide insight into DCAF1 substrate specificity
- PMID: 39580491
- PMCID: PMC11585590
- DOI: 10.1038/s41467-024-54500-x
Crystal structures of DCAF1-PROTAC-WDR5 ternary complexes provide insight into DCAF1 substrate specificity
Abstract
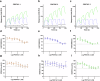
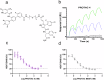
Proteolysis-targeting chimeras (PROTACs) have been explored for the degradation of drug targets for more than two decades. However, only a handful of E3 ligase substrate receptors have been efficiently used. Downregulation and mutation of these receptors would reduce the effectiveness of such PROTACs. We recently developed potent ligands for DCAF1, a substrate receptor of EDVP and CUL4 E3 ligases. Here, we focus on DCAF1 toward the development of PROTACs for WDR5, a drug target in various cancers. We report four DCAF1-based PROTACs with endogenous and exogenous WDR5 degradation effects and high-resolution crystal structures of the ternary complexes of DCAF1-PROTAC-WDR5. The structures reveal detailed insights into the interaction of DCAF1 with various WDR5-PROTACs, indicating a significant role of DCAF1 loops in providing needed surface plasticity, and reflecting the mechanism by which DCAF1 functions as a substrate receptor for E3 ligases with diverse sets of substrates.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures







References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

