Dimethyl fumarate and extracorporeal photopheresis combination-therapy synergize in inducing specific cell death and long-term remission in cutaneous T cell lymphoma
- PMID: 39580583
- PMCID: PMC11794131
- DOI: 10.1038/s41375-024-02479-1
Dimethyl fumarate and extracorporeal photopheresis combination-therapy synergize in inducing specific cell death and long-term remission in cutaneous T cell lymphoma
Abstract
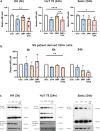
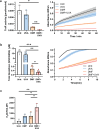
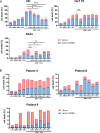
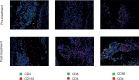
Primary cutaneous T cell lymphomas (CTCL) are characterized by high relapse rates to initially highly effective therapies. Combination therapies have proven beneficial, particularly if they incorporate extracorporeal photopheresis (ECP). The NF-κB inhibitor dimethyl fumarate (DMF) has proven a new, effective drug in CTCL in a clinical phase II study. In vitro experiments with patient-derived SS cells and the CTCL cell lines HH, HuT 78, and SeAx revealed a synergistic effect of DMF and ECP on cell death induction in CTCL cells. Furthermore, an additional increase in the capacity to inhibit NF-κB in CTCL was detected for the combination treatment compared to DMF monotherapy. The same synergistic effects could be measured for ROS production via decreased Thioredoxin reductase activity and glutathione levels. Consequently, a cell death inhibitor screen indicated that the DMF/ECP combination treatment induces a variety of cell death mechanisms in CTCL. As a first step into clinical translation, 4 patients were already treated with the DMF/ECP combination therapy with an overall response rate of 100% and a time to next treatment in skin and blood of up to 57 months. Therefore, our study introduces the combination treatment of DMF and ECP as a highly effective and long-lasting CTCL therapy.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: SM received honoraria and travel funding by Kyowa Kirin. KG received consulting fees from Biogen and supports BioMed X as an academic mentor. JSU is on the advisory board or has received honoraria and travel support from Amgen, Bristol Myers Squibb, GSK, Immunocore, LeoPharma, Merck Sharp and Dohme, Novartis, Pierre Fabre, Roche, Sanofi outside the submitted work. JPN received travel and congress participation funding by TEVA and Novartis as well as consulting fees by TEVA, Almirall, Biogen, Novartis, Kyowa Kirin, Innate Pharma, Takeda and Actelion, UCB Pharma and Recordati. ÖÇŞ, LT, PLB, JDA, TH, DT, and SG have no conflict of interest to declare.
Figures

References
-
- Latzka J, Assaf C, Bagot M, Cozzio A, Dummer R, Guenova E, et al. EORTC consensus recommendations for the treatment of mycosis fungoides/Sezary syndrome - Update 2023. Eur J Cancer. 2023;195:113343. - PubMed
-
- Willemze R, Hodak E, Zinzani PL, Specht L, Ladetto M, Committee EG. Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv30–iv40. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

