AMPK regulates Bcl2-L-13-mediated mitophagy induction for cardioprotection
- PMID: 39580803
- PMCID: PMC11672683
- DOI: 10.1016/j.celrep.2024.115001
AMPK regulates Bcl2-L-13-mediated mitophagy induction for cardioprotection
Abstract
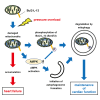
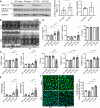
The accumulation of damaged mitochondria in the heart is associated with heart failure. Mitophagy is an autophagic degradation system that specifically targets damaged mitochondria. We have reported previously that Bcl2-like protein 13 (Bcl2-L-13) mediates mitophagy and mitochondrial fission in mammalian cells. However, the in vivo function of Bcl2-L-13 remains unclear. Here, we demonstrate that Bcl2-L-13-deficient mice and knockin mice, in which the phosphorylation site (Ser272) on Bcl2-L-13 was changed to Ala, showed left ventricular dysfunction in response to pressure overload. Attenuation of mitochondrial fission and mitophagy led to impairment of ATP production in these mouse hearts. In addition, we identified AMPKα2 as the kinase responsible for the phosphorylation of Bcl2-L-13 at Ser272. These results indicate that Bcl2-L-13 and its phosphorylation play an important role in maintaining cardiac function. Furthermore, the amplitude of stress-stimulated mitophagic activity could be modulated by AMPKα2.
Keywords: Bcl2-L-13; CP: Cell biology; heart failure; mitochondria; mitophagy.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures






References
-
- McDonagh T.A., Metra M., Adamo M., Gardner R.S., Baumbach A., Böhm M., Burri H., Butler J., Čelutkienė J., Chioncel O., et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Rev. Esp. Cardiol. 2022;75:523. doi: 10.1016/j.rec.2022.05.005. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

