RSL3 sensitizes glioma cells to ionizing radiation by suppressing TGM2-dependent DNA damage repair and epithelial-mesenchymal transition
- PMID: 39580966
- PMCID: PMC11625373
- DOI: 10.1016/j.redox.2024.103438
RSL3 sensitizes glioma cells to ionizing radiation by suppressing TGM2-dependent DNA damage repair and epithelial-mesenchymal transition
Abstract
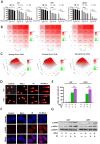
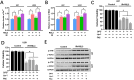
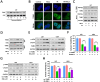
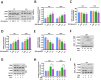
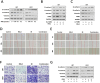
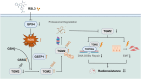
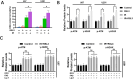
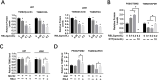
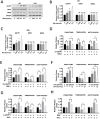
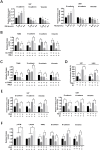
RAS-selective lethal small molecule 3 (RSL3) is a small-molecule compound that triggers ferroptosis by inactivating glutathione peroxidase 4. However, its effect on the radioresistance of glioma cells and the underlying mechanisms remains unclear. In this study, we found that RSL3 sensitized glioma cells to ionizing radiation (IR) and enhanced IR-induced DNA double-strand breaks (DSBs). Inhibition of ferroptosis pathways partly prevented the clonogenic death caused by the IR/RSL3 combination but did not alleviate the DNA DSBs, indicating that RSL3 promotes IR-induced DNA DSBs via a non-ferroptotic mechanism. We demonstrated that transglutaminase 2 (TGM2) plays a vital role in the radiosensitization effect of RSL3 on glioma cells. Treatment with RSL3 downregulated TGM2 in a dose-dependent manner. Overexpression of TGM2 not only alleviated DNA DSBs but also inhibited clonogenic death caused by the IR/RSL3 combination. Mechanistically, RSL3 triggered oxidative stress in glioma cells, which promoted the S-gluthathionylation of TGM2 via upregulation of glutathione S-transferase P1(GSTP1), culminating in the proteasomal degradation of TGM2. This process resulted in the suppression of DNA repair mechanisms by impeding the nuclear accumulation of TGM2 and disrupting the interaction between TGM2 and topoisomerase IIα after irradiation. We also found that RSL3 inhibited glioma cell epithelial-mesenchymal transition (EMT) in both IR-treated and non-IR-treated cells. Overexpression of TGM2 prevented, while knockdown of TGM2 aggravated the EMT inhibition caused by RSL3, indicating that RSL3 also sensitized glioma cells to IR by inhibiting EMT via a TGM2-dependent mechanism. Furthermore, in mice bearing human U87 tumor xenografts, RSL3 administration synergized with IR to inhibit tumor growth, accompanied by TGM2 inhibition, DNA DSBs, and EMT inhibition in tumor tissues. Taken together, we demonstrated that RSL3 sensitizes glioma cells to IR by suppressing TGM2-mediated DNA repair and EMT.
Keywords: Glioma; Oxidative stress; RAS-Selective lethal small molecule 3; S-Glutathionylation; Transglutaminase 2.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
RSL3 triggers glioma stem cell differentiation via the Tgm2/AKT/ID1 signaling axis.Biochim Biophys Acta Mol Basis Dis. 2022 Dec 1;1868(12):166529. doi: 10.1016/j.bbadis.2022.166529. Epub 2022 Aug 27. Biochim Biophys Acta Mol Basis Dis. 2022. PMID: 36041715
-
Transglutaminase 2 promotes epithelial-to-mesenchymal transition by regulating the expression of matrix metalloproteinase 7 in colorectal cancer cells via the MEK/ERK signaling pathway.Biochim Biophys Acta Mol Basis Dis. 2025 Jan;1871(1):167538. doi: 10.1016/j.bbadis.2024.167538. Epub 2024 Oct 9. Biochim Biophys Acta Mol Basis Dis. 2025. PMID: 39389321
-
BMS-345541 sensitizes MCF-7 breast cancer cells to ionizing radiation by selective inhibition of homologous recombinational repair of DNA double-strand breaks.Radiat Res. 2013 Feb;179(2):160-70. doi: 10.1667/RR3034.1. Epub 2012 Dec 21. Radiat Res. 2013. PMID: 23259762 Free PMC article.
-
DNA double strand break repair inhibition as a cause of heat radiosensitization: re-evaluation considering backup pathways of NHEJ.Int J Hyperthermia. 2008 Feb;24(1):17-29. doi: 10.1080/02656730701784782. Int J Hyperthermia. 2008. PMID: 18214766 Review.
-
DNA Damage Clustering after Ionizing Radiation and Consequences in the Processing of Chromatin Breaks.Molecules. 2022 Feb 24;27(5):1540. doi: 10.3390/molecules27051540. Molecules. 2022. PMID: 35268641 Free PMC article. Review.
Cited by
-
A comprehensive bibliometric analysis of ferroptosis in tumor resistance: development and emerging trends.Front Immunol. 2025 May 9;16:1580222. doi: 10.3389/fimmu.2025.1580222. eCollection 2025. Front Immunol. 2025. PMID: 40416987 Free PMC article.
References
-
- Chen X., Kang R., Kroemer G., Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021;18(5):280–296. - PubMed
-
- Ye L.F., Chaudhary K.R., Zandkarimi F., Harken A.D., Kinslow C.J., Upadhyayula P.S., Dovas A., Higgins D.M., Tan H., Zhang Y., Buonanno M., Wang T.J.C., Hei T.K., Bruce J.N., Canoll P.D., Cheng S.K., Stockwell B.R. Radiation-induced lipid peroxidation triggers ferroptosis and synergizes with ferroptosis inducers. ACS Chem. Biol. 2020;15(2):469–484. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

