Dorsal root ganglia CSF1+ neuronal subtypes have different impact on macrophages and microglia after spared nerve injury
- PMID: 39581686
- PMCID: PMC11625985
- DOI: 10.1111/jns.12674
Dorsal root ganglia CSF1+ neuronal subtypes have different impact on macrophages and microglia after spared nerve injury
Abstract
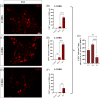
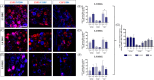
Background and aims: Colony-stimulating factor 1 (CSF1) is a growth factor secreted by dorsal root ganglia (DRG) neurons important for DRG macrophages and spinal cord (SC) microglia injury-induced proliferation and activation, specifically released after spared nerve injury (SNI). In this study, we investigated if SNI-induced CSF1 expression and perineuronal rings of macrophages around mouse DRG neurons vary between L3-L5 DRG and with the neuronal type, and if the CSF1+ neuronal projections at the SC dorsal horns were associated with an increased microglial number in the corresponding laminae.
Methods: Seven days after surgery, L3-L5 DRG as well as their corresponding segments at the SC level were collected, frozen, and cut. DRG sections were double-immunostained using antibodies against CSF1 and NF200, CGRP or IB4, while SC sections were immunostained using a fluorescent Nissl Stain and analyzed for CX3CR1-GFP microglia number and distribution by an in-house ImageJ Plug-in.
Results: Our results showed that SNI-induced CSF1 expression was common for all subtypes of mouse DRG neurons, being responsible for attracting more resident macrophages around them in a DRG-dependent manner, with L4 showing the stronger response and CSF1+/NF200+ neurons showing the highest incidence. Even though the total number of microglia in the SC ipsilateral dorsal horns increased after SNI, the increase at their specific laminar projection sites did not mirror the incidence of DRG neuronal subtypes among CSF1+ neurons.
Interpretation: Taken together, these results contribute to a more comprehensive understanding of the connection between CSF1 and macrophage/microglia response after SNI and emphasize the importance of considering L3-L5 DRG individually when investigating SNI-neuropathic pain pathogenesis in mice.
Keywords: CSF1; macrophages; microglia; neuropathic pain.
© 2024 The Author(s). Journal of the Peripheral Nervous System published by Wiley Periodicals LLC on behalf of Peripheral Nerve Society.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures





References
-
- Calvo M, Dawes JM, Bennett DL. The role of the immune system in the generation of neuropathic pain. Lancet Neurol. 2012;11:629‐642. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

