Stability of symptom-based subtypes in Sjogren's disease
- PMID: 39581689
- PMCID: PMC11590857
- DOI: 10.1136/rmdopen-2024-004914
Stability of symptom-based subtypes in Sjogren's disease
Abstract
Objectives: The Newcastle Sjogren's Stratification Tool (NSST) stratifies Sjogren's disease patients into four subtypes. Understanding the stability of the subtypes is vital if symptom-based stratification is to be more broadly adopted. In this study, we stratify patients longitudinally to understand how symptom-based subtypes vary over time and factors influencing subtype change.
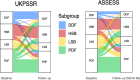
Methods: 274 patients from the United Kingdom Primary Sjögren's Syndrome Registry (UKPSSR) with data permitting NSST subtype assignment from two study visits were included. The French Assessment of Systemic Signs and Evolution of Sjogren's Syndrome (ASSESS) cohort (n=237) acted as an independent comparator. Group analyses of significant differences were performed, with logistic regression models used to assess covariates of subtype stability.
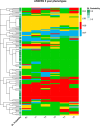
Results: UKPSSR and ASSESS cohorts showed a broadly similar proportion of subjects in each subtype and similar baseline clinical characteristics except body mass index (BMI). Several baseline characteristics differ significantly between the subtypes, most notably anti-Ro status and BMI. Subtype membership was reasonably stable in both cohorts with 60% and 57% retaining subtype. The high-symptom burden subtype was the most stable over time with 70% and 67% retaining subtype. Higher baseline probability score was the greatest predictor of subtype stability with higher C4 levels, antidepressant use, and a higher CCI score also predicting increased stability.
Conclusion: NSST subtype membership remains stable over time in a large proportion of patients. When subtype transition is associated with factors at baseline, it is most strongly associated with an uncertain subtype allocation. Our findings support the hypothesis that symptom-based subtypes reflect genuine pathobiological endotypes and therefore maybe important to consider in trial design and clinical management.
Keywords: Autoantibodies; Classification; Fatigue; Patient Reported Outcome Measures; Sjogren's Syndrome.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: XM received consultant fees from BMS, Galapagos, GSK, Novartis and Pfizer. W-FN has undertaken clinical trials and provided consultancy or expert advice in the area of Sjögren’s syndrome to the following companies: GlaxoSmithKline, MedImmune, UCB, Abbvie, Takeda, Resolves Therapeutics, Sanofi, Novartis, Bain Capitals and Argenx. No other potential conflict of interest relevant to this article was reported.
Figures
References
-
- Colafrancesco S, Priori R. Sjögren’s Disease: A New Era for Clinical Trials. Vol. 404. The Lancet. Elsevier B.V; 2024. pp. 498–9. - PubMed
-
- Tarn JR, Howard-Tripp N, Lendrem DW, et al. Symptom-based stratification of patients with primary Sjögren’s syndrome: multi-dimensional characterisation of international observational cohorts and reanalyses of randomised clinical trials. Lancet Rheumatol. 2019;1:e85–94. doi: 10.1016/S2665-9913(19)30042-6. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
