Propafenone facilitates mitochondrial-associated ferroptosis and synergizes with immunotherapy in melanoma
- PMID: 39581704
- PMCID: PMC11590812
- DOI: 10.1136/jitc-2024-009805
Propafenone facilitates mitochondrial-associated ferroptosis and synergizes with immunotherapy in melanoma
Abstract
Background: Despite the successful application of immunotherapy, both innate and acquired resistance are typical in melanoma. Ferroptosis induction appears to be a potential strategy to enhance the effectiveness of immunotherapy. However, the relationship between the status of ferroptosis and the effectiveness of immunotherapy, as well as viable strategies to augment ferroptosis, remains unclear.
Methods: A screening of 200 cardiovascular drugs obtained from the Food and Drug Administration-approved drug library was conducted to identify the potential ferroptosis sensitizer. In vitro and in vivo experiments explored the effects of propafenone on ferroptosis in melanoma. Animal models and transcriptomic analyses evaluated the therapeutic effects and survival benefits of propafenone combined with immune checkpoint blockades (ICBs). The relationship between propafenone targets and the efficacy of ICBs was validated using the Xiangya melanoma data set and publicly available clinical data sets.
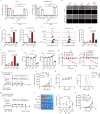
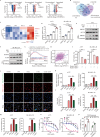
Results: Through large-scale drug screening of cardiovascular drugs, we identified propafenone, an anti-arrhythmia medication, as capable of synergizing with ferroptosis inducers in melanoma. Furthermore, we observed that propafenone, in combination with glutathione peroxidase 4 inhibitor RSL3, collaboratively induces mitochondrial-associated ferroptosis. Mechanistically, propafenone transcriptionally upregulates mitochondrial heme oxygenase 1 through the activation of the Jun N-terminal kinase (JNK)/JUN signaling pathway under RSL3 treatment, leading to overloaded ferrous iron and reactive oxygen species within the mitochondria. In xenograft models, the combination of propafenone and ferroptosis induction led to nearly complete tumor regression and prolonged survival. Consistently, propafenone enhances immunotherapy-induced tumorous ferroptosis and antitumor immunity in tumor-bearing mice. Significantly, patients exhibiting high levels of ferroptosis/JUN/HMOX1 exhibited improved efficacy of immunotherapy and prolonged progression-free survival.
Conclusions: Taken together, our findings suggest that propafenone holds promise as a candidate drug for enhancing the efficacy of immunotherapy and other ferroptosis-targeted therapies in the treatment of melanoma.
Keywords: Immunotherapy; Melanoma.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures





References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
