Pemafibrate for treating MASLD complicated by hypertriglyceridaemia: a multicentre, open-label, randomised controlled trial study protocol
- PMID: 39581726
- PMCID: PMC11590823
- DOI: 10.1136/bmjopen-2024-088862
Pemafibrate for treating MASLD complicated by hypertriglyceridaemia: a multicentre, open-label, randomised controlled trial study protocol
Abstract
Introduction: Non-alcoholic fatty liver disease, now known as metabolic dysfunction-associated steatotic liver disease (MASLD), is a phenotype of the metabolic syndrome in the liver and is clearly associated with metabolic abnormalities such as hyperglycaemia and dyslipidaemia. Although the prevalence of MASLD is increasing worldwide, there is currently no consensus on the efficacy and safety of the drugs used to treat MASLD/metabolic dysfunction-associated steatohepatitis (MASH). Pemafibrate, a selective peroxisome proliferator-activated receptor alpha modulator, was designed to have higher peroxisome proliferator-activated receptor alfa (PPARα) agonist activity and selectivity than existing PPARα agonists, and in development trials, without increasing creatinine levels, lipid parameters and alanine aminotransferase (ALT) were significantly improved. Thus, pemafibrate may effectively ameliorate the pathogenesis and metabolic abnormalities in MASLD/MASH. In this trial, we evaluated the efficacy and safety of pemafibrate in patients with MASLD/MASH.
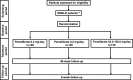
Methods and analysis: This trial was designed as an open-label, three-arm, randomised controlled study. After obtaining informed consent, patients aged 20-80 years who met the selection criteria were enrolled. Patients were randomised to receive pemafibrate 0.4 mg/day, 0.2 mg/day or fenofibrate (n=120 per group). The duration of treatment was 48 weeks. The primary endpoint was a change in ALT levels after 24 weeks of administration. Secondary endpoints included changes from baseline in liver fibrosis markers (fibrosis-4 index, type IV collagen 7s, enhanced liver fibrosis and Mac-2 binding protein glycosylation isomer) at 48 weeks as well as changes in liver fat mass and liver stiffness measured by MRI and ultrasound (US) at centres equipped with MRI and US capabilities.
Ethics and dissemination: Ethical approval was obtained from the Yokohama City University Certified Institutional Review Board before participant enrolment (CRB20-014). The results of this study will be submitted for publication in international peer-reviewed journals and the key findings will be presented at international scientific conferences. Participants wishing to understand the results of this study will be contacted directly on data publication.
Trial registration number: This trial was registered in the Japan Registry of Clinical Trials (number: jRCTs031200280).
Protocol version: V.1.9, 23 November 2023.
Keywords: Hepatology; Lipid disorders; Other metabolic, e.g. iron, porphyria.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: ANa received grants and research support from Gilead, Mylan EPD, EA Pharma, Kowa, Taisho and Biofermin. ANa is a consulting advisor for Gilead, Boehringer Ingelheim, BMS, Kowa, Astellas, EA Pharma and Mylan EPD. The other authors declare no conflict of interest.
Figures
References
-
- Kanwal F, Neuschwander-Tetri BA, Loomba R, et al. Metabolic dysfunction-associated steatotic liver disease: Update and impact of new nomenclature on the American Association for the Study of Liver Diseases practice guidance on nonalcoholic fatty liver disease. Hepatology. 2024;79:1212–9. doi: 10.1097/HEP.0000000000000670. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous