Effects of smartphone-based chatbot intervention to increase influenza and COVID-19 vaccine uptake among South Asian ethnic minorities in Hong Kong: protocol for a randomised waitlist-controlled trial
- PMID: 39581736
- PMCID: PMC11789508
- DOI: 10.1136/bmjopen-2023-080725
Effects of smartphone-based chatbot intervention to increase influenza and COVID-19 vaccine uptake among South Asian ethnic minorities in Hong Kong: protocol for a randomised waitlist-controlled trial
Abstract
Introduction: Protecting non-native ethnic minority groups against cocirculation of influenza and COVID-19 is crucial, and vaccination could be a viable option. Smartphone-based chatbots offer promising opportunities for improving vaccine knowledge and addressing barriers encountered by ethnic minorities. This trial aims to evaluate the effects of smartphone-based chatbot intervention on influenza and COVID-19 vaccine uptake, intention to receive vaccination and vaccine hesitancy among South Asian ethnic minorities in Hong Kong.
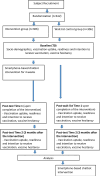
Method and analysis: An assessor-blinded, cluster-randomised, waitlist-controlled trial will be conducted. This study consists of two phases. In phase I, a smartphone-based chatbot intervention will be developed, including designing a simple chatbot called 'Ali' to deliver prewritten educational text messages and vaccination reminders as well as respond to users' questions, and on-demand option for communication with research assistants. An expert panel will be invited to review the designed chatbot, and pilot testing will be performed. In phase II, a total of 612 South Asians will be recruited from each of the six participating non-governmental community centres or ethnic minority associations. They will be randomly allocated to intervention (n=306) or waitlist control group (n=306). The intervention group will receive in-app notifications related to the education text messages and vaccination reminders via the smartphone-based chatbot twice a week for two weeks. The waitlist control group will receive usual care only. Evaluation will include vaccination uptake, intention to receive vaccination and vaccine hesitancy. Assessments will take place at baseline (T0), immediately postintervention (T1) and 3-month postintervention (T2).
Ethics and dissemination: This study has been approved by the Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee (CREC Ref. No.: 2021.688). The findings will be disseminated in peer-reviewed journals and through local or interventional conference presentations.
Trial registration number: ChiCTR2200061503.
Keywords: COVID-19; Health Education; Nursing Care; PUBLIC HEALTH; Randomized Controlled Trial; Vaccination.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Multilevel Determinants of COVID-19 Vaccine Uptake Among South Asian Ethnic Minorities in Hong Kong: Cross-sectional Web-Based Survey.JMIR Public Health Surveill. 2021 Nov 9;7(11):e31707. doi: 10.2196/31707. JMIR Public Health Surveill. 2021. PMID: 34653014 Free PMC article.
-
Nudging towards COVID-19 and influenza vaccination uptake in medically at-risk children: EPIC study protocol of randomised controlled trials in Australian paediatric outpatient clinics.BMJ Open. 2024 Feb 17;14(2):e076194. doi: 10.1136/bmjopen-2023-076194. BMJ Open. 2024. PMID: 38367966 Free PMC article.
-
Editor's Choice: Influenza vaccine uptake, COVID-19 vaccination intention and vaccine hesitancy among nurses: A survey.Int J Nurs Stud. 2021 Feb;114:103854. doi: 10.1016/j.ijnurstu.2020.103854. Epub 2020 Dec 5. Int J Nurs Stud. 2021. PMID: 33326864 Free PMC article.
-
Interventions to increase COVID-19 vaccine uptake: a scoping review.Cochrane Database Syst Rev. 2022 Aug 3;8(8):CD015270. doi: 10.1002/14651858.CD015270. Cochrane Database Syst Rev. 2022. PMID: 35920693 Free PMC article.
-
Influenza virus vaccine live intranasal--MedImmune vaccines: CAIV-T, influenza vaccine live intranasal.Drugs R D. 2003;4(5):312-9. doi: 10.2165/00126839-200304050-00007. Drugs R D. 2003. PMID: 12952502 Review.
References
-
- Centre for Health Protection Recommendation on seasonal influenza vaccination for the 2020-21 season in Hong Kong. 2020. https://www.chp.gov.hk/en/static/24008.html Available.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical