Pneumococci remain the main cause of complicated pediatric pneumonia in the post-pandemic era despite extensive pneumococcal vaccine use
- PMID: 39582053
- PMCID: PMC11587768
- DOI: 10.1186/s41479-024-00151-x
Pneumococci remain the main cause of complicated pediatric pneumonia in the post-pandemic era despite extensive pneumococcal vaccine use
Erratum in
-
Correction to: Pneumococci remain the main cause of complicated pediatric pneumonia in the post-pandemic era despite extensive pneumococcal vaccine use.Pneumonia (Nathan). 2025 Sep 15;17(1):25. doi: 10.1186/s41479-025-00179-7. Pneumonia (Nathan). 2025. PMID: 40954506 Free PMC article. No abstract available.
Abstract
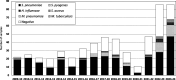
Nucleic acid amplification tests (NAATs) greatly enhance the capacity to identify the etiology of pediatric complicated pneumonia. However, the use of pneumococcal conjugate vaccines could reduce the importance of Streptococcus pneumoniae in pediatric complicated pneumonia with the potential emergence of other bacterial agents. Using an expanded NAAT in culture negative pleural fluid or empyema samples collected in 2010-2024 (n = 554) in Portugal, we show that S. pneumoniae remains the most frequent agent despite decades of pneumococcal conjugate vaccine use and the COVID-19 pandemic. A rebound in pediatric complicated pneumonia occurred post-pandemic, including a rise in cases by Streptococcus pyogenes and Haemophilus influenzae. Empiric therapy of pediatric complicated pneumonia should still consider S. pneumoniae as the most likely cause, even in countries where the pneumococcal conjugate vaccine is in the national immunization program with a high uptake.
Keywords: Empyema; Epidemiology; Molecular Diagnostics; Pediatric infectious disease; Serotypes; Streptococcus pneumonia; Vaccines.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: No personal and sensitive data was collected. The data collected is anonymous data, i.e., the identity of the person to whom the data are referred to was unknown. The patient samples are collected within the context of the diagnostic workup at the discretion of the attending physician and no specific guidelines or recommendations are in force because of the study. The study was approved by the Institutional Review Board of the Centro Académico de Medicina de Lisboa (240/22). Since these were considered surveillance activities they were exempt from informed consent. Consent for publication: Not applicable. Competing interests: JM-C received research grants administered through his university and received honoraria for serving on the speakers bureaus of Pfizer and Merck Sharp and Dohme. MR received honoraria for serving on the speakers bureau of Pfizer and Merck Sharp and Dohme, for serving in expert panels of Merck Sharp and Dohme, support for attending meetings from Pfizer, and received research grants administered through his university from Merck Sharp and Dohme. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Silva-Costa C, Brito MJ, Pinho MD, Friães A, Aguiar SI, Ramirez M, et al. Pediatric Complicated Pneumonia Caused by Streptococcus pneumoniae Serotype 3 in 13-Valent Pneumococcal Conjugate Vaccinees, Portugal, 2010–2015. Emerging Infect Dis. 2018;24:1307–14. 10.3201/eid2407.180029. - DOI - PMC - PubMed
-
- Selva L, Ciruela P, Esteva C, de Sevilla MF, Codina G, Hernandez S, et al. Serotype 3 is a common serotype causing invasive pneumococcal disease in children less than 5 years old, as identified by real-time PCR. Eur J Clin Microbiol Infect Dis. 2012;31:1487–95. 10.1007/s10096-011-1468-7. - DOI - PubMed
-
- Teresinha Mocelin H, Bueno Fischer G, Danezi Piccini J, de Oliveira Espinel J, Feijó Andrade C, Bush A. Necrotizing Pneumonia In Children: A Review. Paediatric Respiratory Reviews. 2024. 10.1016/j.prrv.2024.02.003. [Cited 1 Jul 2024] - PubMed
LinkOut - more resources
Full Text Sources