Enhancing mitochondrial one-carbon metabolism is neuroprotective in Alzheimer's disease models
- PMID: 39582067
- PMCID: PMC11586400
- DOI: 10.1038/s41419-024-07179-3
Enhancing mitochondrial one-carbon metabolism is neuroprotective in Alzheimer's disease models
Abstract
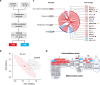
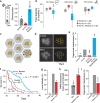
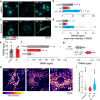
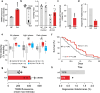
Alzheimer's disease (AD) is the most common form of age-related dementia. In AD, the death of neurons in the central nervous system is associated with the accumulation of toxic amyloid β peptide (Aβ) and mitochondrial dysfunction. Mitochondria are signal transducers of metabolic and biochemical information, and their impairment can compromise cellular function. Mitochondria compartmentalise several pathways, including folate-dependent one-carbon (1C) metabolism and electron transport by respiratory complexes. Mitochondrial 1C metabolism is linked to electron transport through complex I of the respiratory chain. Here, we analysed the proteomic changes in a fly model of AD by overexpressing a toxic form of Aβ (Aβ-Arc). We found that expressing Aβ-Arc caused alterations in components of both complex I and mitochondrial 1C metabolism. Genetically enhancing mitochondrial 1C metabolism through Nmdmc improved mitochondrial function and was neuroprotective in fly models of AD. We also found that exogenous supplementation with the 1C donor folinic acid improved mitochondrial health in both mammalian cells and fly models of AD. We found that genetic variations in MTHFD2L, the human orthologue of Nmdmc, were linked to AD risk. Additionally, Mendelian randomisation showed that increased folate intake decreased the risk of developing AD. Overall, our data suggest enhancement of folate-dependent 1C metabolism as a viable strategy to delay the progression and attenuate the severity of AD.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: YY is an inventor on a filed patent that is related to enhancing folate one-carbon metabolism to protect against Alzheimer’s disease. YY is a founder and holds equity in Healthspan Biotics.
Figures



References
-
- Schwarzinger M, Dufouil C. Forecasting the prevalence of dementia. Lancet Public Health. 2022;7:e94–e95. - PubMed
-
- Selkoe DJ. The molecular pathology of Alzheimer’s disease. Neuron. 1991;6:487–98. - PubMed
-
- Levy E, Carman MD, Fernandez-Madrid IJ, Power MD, Lieberburg I, van Duinen SG, et al. Mutation of the Alzheimer’s disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science. 1990;248:1124–6. - PubMed
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

