Identification of a Nuclear Localization Signal (NLS) in Human Transcription Elongation Factor ELL2
- PMID: 39582094
- PMCID: PMC11586470
- DOI: 10.1002/cbf.70019
Identification of a Nuclear Localization Signal (NLS) in Human Transcription Elongation Factor ELL2
Abstract
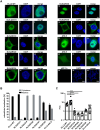
ELL2 is a transcription elongation factor suppressing transcriptional pausing of RNA polymerase II, thereby enhancing gene expression. In accordance with the nuclear localization of ELL2, the protein is supposed to carry out its function in promoting transcription in the nucleus. Yet, it is unknown whether ELL2 carries a nuclear localization signal (NLS). In this study, we identify the NLS of ELL2. In silico analysis resulted in prediction of a strong bipartite NLS with an exceptionally high score at amino acids 311-338 in the conserved region R1 of ELL2. Confocal laser scanning microscopy of a series of ELL2 truncation mutants and quantitative analysis of images verified the presence of R1 to be decisive for nuclear localization of ELL2 suggesting that the predicted NLS is accurate. Deletion of key basic amino acids within the putative NLS in silico and in vitro showed that K319, R320, and K333/K334 are crucial for ELL2's nuclear accumulation, thus confirming the predictions. The isolated ELL2-NLS was able to translocate an unrelated NLS-mapping system into the nucleus underlining the strength of the NLS. Taken together, we identified the NLS of ELL2 and mapped individual aa that are crucial for nuclear localization of ELL2.
Keywords: ELL2; NLS; nuclear localization signal; transcription elongation factor.
© 2024 The Author(s). Cell Biochemistry and Function published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Kohrt S., Strobel S., Mann M., Sticht H., Fleckenstein B., and Thoma‐Kress A., “Characterizing the Interaction Between the HTLV‐1 Transactivator Tax‐1 With Transcription Elongation Factor ELL2 and Its Impact on Viral Transactivation,” International Journal of Molecular Sciences 22 (2021): 13597. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

