A human stem cell-derived model reveals pathologic extracellular matrix remodeling in diabetic podocyte injury
- PMID: 39582511
- PMCID: PMC11585791
- DOI: 10.1016/j.mbplus.2024.100164
A human stem cell-derived model reveals pathologic extracellular matrix remodeling in diabetic podocyte injury
Abstract
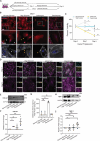
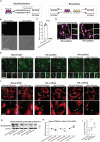
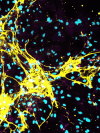
Diabetic nephropathy results from chronic (or uncontrolled) hyperglycemia and is the leading cause of kidney failure. The kidney's glomerular podocytes are highly susceptible to diabetic injury and subsequent non-reversible degeneration. We generated a human induced pluripotent stem (iPS) cell-derived model of diabetic podocytopathy to investigate disease pathogenesis and progression. The model recapitulated hallmarks of podocytopathy that precede proteinuria including retraction of foot processes and podocytopenia (detachment from the extracellular matrix (ECM)). Moreover, hyperglycemia-induced injury to podocytes exacerbated remodeling of the ECM. Specifically, mature podocytes aberrantly increased expression and excessively deposited collagen (IV)α1α1α2 that is normally abundant in the embryonic glomerulus. This collagen (IV) imbalance coincided with dysregulation of lineage-specific proteins, structural abnormalities of the ECM, and podocytopenia - a mechanism not shared with endothelium and is distinct from drug-induced injury. Intriguingly, repopulation of hyperglycemia-injured podocytes on decellularized ECM scaffolds isolated from healthy podocytes attenuated the loss of synaptopodin (a mechanosensitive protein associated with podocyte health). These results demonstrate that human iPS cell-derived podocytes can facilitate in vitro studies to uncover the mechanisms of chronic hyperglycemia and ECM remodeling and guide disease target identification.
Keywords: Collagen (IV); Decellularized extracellular matrix; Diabetic nephropathy; Human induced pluripotent stem cells; Hyperglycemia; Podocytes.
© 2024 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: S.M. is an inventor on a patent regarding podocyte differentiation, U.S. Patent No. 11,083,754 B2. U.S. Patent and Trademark Office. All other authors declare no other competing interests.
Figures




Similar articles
-
Nephrin is necessary for podocyte recovery following injury in an adult mature glomerulus.PLoS One. 2018 Jun 20;13(6):e0198013. doi: 10.1371/journal.pone.0198013. eCollection 2018. PLoS One. 2018. PMID: 29924795 Free PMC article.
-
Mechanosensitive Differentiation of Human iPS Cell-Derived Podocytes.Bioengineering (Basel). 2024 Oct 17;11(10):1038. doi: 10.3390/bioengineering11101038. Bioengineering (Basel). 2024. PMID: 39451413 Free PMC article.
-
Podocyte-Specific Expression of the Stress Response Protein REDD1 Is Necessary for Diabetes-Induced Podocytopenia.Diabetes. 2025 Mar 1;74(3):398-408. doi: 10.2337/db24-0533. Diabetes. 2025. PMID: 39320924 Free PMC article.
-
The Life of a Kidney Podocyte.Acta Physiol (Oxf). 2025 Aug;241(8):e70081. doi: 10.1111/apha.70081. Acta Physiol (Oxf). 2025. PMID: 40698593 Free PMC article. Review.
-
Revisiting nephrin signaling and its specialized effects on the uniquely adaptable podocyte.Biochem J. 2025 Jun 2;482(11):763-88. doi: 10.1042/BCJ20230234. Biochem J. 2025. PMID: 40457651 Free PMC article. Review.
References
-
- Centers for Disease Control and Prevention. Chronic Kidney Disease in the United States, 2023. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2023.
-
- P. Rossing, M.L. Caramori, J.C.N. Chan, H.J.L. Heerspink, C. Hurst, K. Khunti, A. Liew, E.D. Michos, S.D. Navaneethan, W.A. Olowu, T. Sadusky, N. Tandon, K.R. Tuttle, C. Wanner, K.G. Wilkens, S. Zoungas, J.C. Craig, D.J. Tunnicliffe, M.A. Tonelli, M. Cheung, A. Earley, I.H. De Boer, Executive summary of the KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease: an update based on rapidly emerging new evidence, Kidney Int. 102 (2022) 990–999. https://doi.org/10.1016/j.kint.2022.06.013. - PubMed
-
- United States Renal Data System, 2023 USRDS Annual Data Report: Epidemiology of kidney disease in the United States Annual Data Report, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (2023).
Grants and funding
LinkOut - more resources
Full Text Sources

