Association between vaccination and persistent COVID-19-related symptoms among patients with mild Omicron infection: A prospective cohort study
- PMID: 39582794
- PMCID: PMC11582428
- DOI: 10.1016/j.jvacx.2024.100579
Association between vaccination and persistent COVID-19-related symptoms among patients with mild Omicron infection: A prospective cohort study
Erratum in
-
Corrigendum to Association between vaccination and persistent COVID-19-related symptoms among patients with mild Omicron infection: A prospective cohort study.Vaccine X. 2024 Dec 19;22:100603. doi: 10.1016/j.jvacx.2024.100603. eCollection 2025 Jan. Vaccine X. 2024. PMID: 39968079 Free PMC article.
Abstract
Background: While COVID-19 vaccination has been shown to reduce the risk of severe illness, its impact on the occurrence of persistent symptoms in patients with mild Omicron infection remains uncertain. Our objective was to investigate whether COVID-19 vaccination reduces the occurrence of persistent COVID-19-related symptoms 3 months after mild Omicron infection.
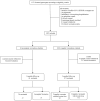
Methods: Multicenter prospective cohort study was conducted in Brazil between January 2022 and June 2023 when Omicron was predominant. Participants ≥ 18 years seeking outpatient care for symptomatic SARS-CoV-2 infection were enrolled. Complete vaccination included individuals who received the full primary series and any booster dose, while incomplete vaccination included those with incomplete primary series or no vaccination. The primary outcome was any persistent symptoms at 3 months. Secondary outcomes were organ system-specific persistent symptoms and the EQ-5D-3L utility score. All outcomes were assessed by means of structured telephone interviews 3 months after enrollment.
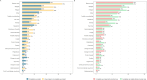
Results: 1,067 patients were enrolled (median age, 39 years), of which 914 (871 completely vaccinated and 43 unvaccinated or incompletely vaccinated). Among the vaccinated participants the median time since the last vaccine dose was 145 (interquartile range, 106-251) days. A total of 388/1067 (36.9 %) had a prior infection at the time of study entry. The occurrence of overall persistent COVID-19-related symptoms at 3 months was 41.6 % (n = 362) among completely vaccinated and 44.2 % (n = 19) among unvaccinated or incompletely vaccinated patients (adjusted risk ratio [aRR], 0.87; 95 % confidence interval [CI], 0.61-1.23; p = 0.43). Complete vaccination was associated with lower occurrence of mental health symptoms (aRR, 0.44; 95 % CI, 0.24-0.81; p = 0.01). No differences were found in the occurrence of persistent symptoms in other specific domains, nor in EQ-5D-3L utility scores.
Conclusions: This study was not able to identify a statistically significant protection of complete COVID-19 vaccination against any overall persistent symptoms at 3 months. Nevertheless, complete vaccination was associated with a lower occurrence of persistent mental health symptoms.
Keywords: COVID-19 vaccine; COVID-19 vaccine booster shot; Post-Acute COVID-19 Syndrome.
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The authors MMR, FLS, GT, MMDS, DS, RRMS, RFCS, ESR, GPE, GSR, DM, JMBS, APM, CRI, GNS, JMN, HJMF, CVPS, ASS, CMD, CMS, JVP and IFS work at Hospital Moinhos de Vento, which received a research grant from the Brazilian Ministry of Health for the conduction of this study. RGR and MF had received research grants from the Brazilian Ministry of Health, Pfizer and MSD. All other authors declare that they have no conflict of interest. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- World Health Organization. A clinical case definition of post COVID-19 condition by a Delphi consensus, 6 October 2021. apps.who.int [Internet]. 2021. Available from: https://apps.who.int/iris/handle/10665/345824.
-
- WHO. Coronavirus disease (COVID-19): Post COVID-19 condition. www.who.int [Internet]. 2021. Available from: https://www.who.int/news-room/questions-and-answers/item/coronavirus-dis....
-
- Simon M.A., Luginbuhl R.D., Parker R. Reduced incidence of long-COVID symptoms related to administration of COVID-19 vaccines both before COVID-19 diagnosis and up to 12 weeks after. Medrxiv. 2021 Nov 18 doi: 10.1101/2021.11.17.21263608. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous