Impact of SARS-CoV-2 vaccination in patients with vascular liver diseases: Observations from a VALDIG multicenter study
- PMID: 39583091
- PMCID: PMC11582744
- DOI: 10.1016/j.jhepr.2024.101191
Impact of SARS-CoV-2 vaccination in patients with vascular liver diseases: Observations from a VALDIG multicenter study
Abstract
Background & aims: Patients with vascular liver diseases (VLD) are at higher risk of both severe courses of COVID-19 disease and thromboembolic events. The impact of SARS-CoV-2 vaccination in patients with VLD has not been described and represents the aim of our study.
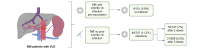
Methods: International, multicenter, prospective observational study in patients with VLD analyzing the incidence of COVID-19 infection after vaccination, severity of side effects, occurrence of thromboembolic events and hepatic decompensation. In a subgroup of patients, the humoral and cellular responses to vaccination were also analyzed.
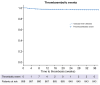
Results: A total of 898 patients from 14 European centers - part of the VALDIG network - were included, 872 (97.1%) patients received two vaccine doses (fully vaccinated), and 674 (75.1%) three doses. Of the total cohort, 151/898 had a COVID-19 infection prior to vaccination, of whom 9/151 (5.9%) were re-infected. Of the 747/898 patients who were not previously infected, 11.2% (84/747) were diagnosed with a COVID-19 infection during the study period. Two infected patients required intensive care unit admission and infection was fatal in two fully vaccinated patients. Adverse effects were reported in around 40% of patients, with local side effects being the most frequent. During the study period, 31 (3.5%) patients had thromboembolic events and 21 (2.3%) hepatic decompensations. No cases of vaccine-induced thrombocytopenia were reported. Vaccine immunogenicity was assessed in 36 patients; seroconversion reached 100% and IFNy T-cell responses significantly increased post two mRNA-1273 vaccine doses.
Conclusion: Patients with VLD seem to have a preserved immune response to SARS-CoV-2 vaccination, which appears to be safe and effective in preventing severe COVID-19 infection. Our study cannot definitively establish a direct link between vaccination and thrombotic events, though the contribution of vaccination as a cofactor in VLD remains to be elucidated.
Impact and implications: Patients with vascular liver disease (VLD) are at increased risk of both SARS-CoV-2 infection and severe COVID-19 disease. The potential risks associated with vaccination against this infection need thorough investigation. Our research enhances the understanding of the effects of COVID-19 vaccination in patients with VLD, highlighting its good tolerability. Moreover, patients with VLD appear to have a preserved immune response to SARS-CoV-2 vaccination, providing protection against severe COVID-19 infection. Our study cannot definitively establish a direct link between vaccination and thrombotic events, and no cases of vaccine-induced thrombocytopenia were reported.
Keywords: COVID-19 vaccine; Vascular liver disease; portal thrombosis.
© 2024 The Authors.
Figures


References
-
- European Association for the Study of the Liver Electronic address: easloffice@easloffice.eu. EASL Clinical Practice Guidelines: vascular diseases of the liver. J Hepatol. 2016 Jan;64(1):179–202. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

