Targeted Delivery and ROS-Responsive Release of Lutein Nanoassemblies Inhibit Myocardial Ischemia-Reperfusion Injury by Improving Mitochondrial Function
- PMID: 39583319
- PMCID: PMC11585303
- DOI: 10.2147/IJN.S488532
Targeted Delivery and ROS-Responsive Release of Lutein Nanoassemblies Inhibit Myocardial Ischemia-Reperfusion Injury by Improving Mitochondrial Function
Abstract
Purpose: Myocardial ischemia-reperfusion injury (MI/RI) is associated with increased oxidative damage and mitochondrial dysfunction, resulting in an elevated risk of mortality. MI/RI may be alleviated by protecting cardiomyocytes from oxidative stress. Lutein, which belongs to a class of carotenoids, has proven to be effective in cardiovascular disease treatment due to its remarkable antioxidant properties, but its application is limited due to its poor stability and low bioavailability in vivo.
Methods: In this study, a delivery system was developed based on distearoyl phosphatidyl ethanolamine (DSPE)-thiol-ketone (TK)-PEG2K (polyethylene glycol 2000) (abbreviated as DTP) and PCM-SH (CWLSEAGPVVTVRALRGTGSW) to deliver lutein (abbreviated as lutein@DTPP) to damaged myocardium. First, lutein, lutein@DTP, or lutein@DTPP were injected through the tail vein once a day for 3 days and then MI/RI model rats were established by exposing rats to ischemia for 45 min and reperfusion for 6 h. We employed a range of experimental techniques including qRT-PCR, Western blotting, transmission electron microscopy, immunohistochemistry, immunofluorescence, flow cytometry, immunoprecipitation, molecular docking, and molecular dynamics simulations.
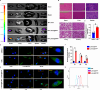
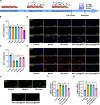
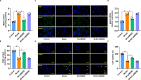
Results: Lutein@DTPP exhibited good myocardial targeting and ROS-responsive release. Our data suggested that lutein@DTPP effectively suppresses ferroptosis in cardiomyocytes. Mechanistically, we observed an upregulation of mouse double minute-2 (MDM2) in the hearts of MI/RI models and cardiomyocytes exposed to hypoxia/reoxygenation (H/R) conditions. In addition, NADH-ubiquinone oxidoreductase 75 kDa Fe-S protein 1 (NDUFS1) translocation from the cytosol to the mitochondria was inhibited by MDM2 upregulation. Notably, no significant variation in the total NDUFS1 expression was observed in H/R-exposed cardiomyocytes following treatment with siMDM2. Further study indicated that lutein facilitates the translocation of NDUFS1 from the cytosol to mitochondria by directly binding and sequestering MDM2, thereby improving mitochondrial function and inhibiting ferroptosis.
Conclusion: Lutein@DTPP promoted the mitochondrial translocation of NDUFS1 to restore mitochondrial function and inhibited the ferroptosis of cardiomyocytes by directly binding and sequestering MDM2.
Keywords: MDM2; NDUFS1; ROS; ferroptosis; lutein; myocardial ischemia-reperfusion injury.
© 2024 Shi et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

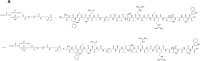







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

