Curcumin-Loaded Long-Circulation Liposomes Ameliorate Insulin Resistance in Type 2 Diabetic Mice
- PMID: 39583326
- PMCID: PMC11585265
- DOI: 10.2147/IJN.S487519
Curcumin-Loaded Long-Circulation Liposomes Ameliorate Insulin Resistance in Type 2 Diabetic Mice
Abstract
Introduction: Type 2 diabetes mellitus (T2DM) is a metabolic disorder characterised by insulin resistance, hyperglycaemia, and inflammation, with oxidative stress contributing to its progression. Curcumin (CUR), known for its anti-inflammatory, antioxidant, and insulin sensitising effects, has shown potential for the treatment of T2DM but is limited by low solubility and bioavailability. This study investigated long-circulating curcumin-loaded liposomes (CUR-LPs) to improve curcumin stability, solubility, and circulation and assessed their effect on insulin resistance in a murine model of T2DM.
Methods: CUR-LPs were prepared using the ethanol injection method and characterized for morphology, particle size, zeta potential, encapsulation efficiency, drug-loading capacity, and in vitro release. Cell viability was tested on murine L929 cells. In a T2DM murine model, after four weeks of CUR-LP treatment, inflammatory markers TNF-α and IL-6 were measured by real-time polymerase chain reaction, and liver tissues were analyzed for glutathione (GSH) and superoxide dismutase (SOD) via colorimetry.
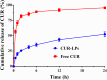
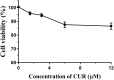
Results: CUR-LPs were spherical, with an average diameter of (249 ± 2.3) nm and a zeta potential of (-33.5 ± 0.8) mV. They exhibited an encapsulation efficiency of (99.2 ± 0.5) %and a drug-loading capacity of (1.63 ± 0.02) %. CUR embedding in liposomes significantly maintained CUR release. In L929 cells, over 80% viability was maintained at 12 uM CUR concentration after 24 h. In HFD/STZ-induced T2DM mice, CUR-LPs improved blood glucose and insulin levels more efficiently than free CUR, and CUR-LPs also reduced hepatic inflammation (TNF-α, IL-6), enhanced hepatic GSH and SOD, and attenuated liver injury.
Conclusion: CUR-LPs improved glucose metabolism and insulin resistance in HFD/STZ-induced T2DM mice, which may be associated with a decrease in liver inflammation and oxidative stress.
Keywords: T2DM; curcumin; glucose metabolism; insulin resistance; liposomes; oxidative stress.
© 2024 Li et al.
Conflict of interest statement
The authors declared no conflict of interest.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

