Elevated tau in the piriform cortex in Alzheimer's but not Parkinson's disease using PET-MR
- PMID: 39583648
- PMCID: PMC11585164
- DOI: 10.1002/dad2.70040
Elevated tau in the piriform cortex in Alzheimer's but not Parkinson's disease using PET-MR
Abstract
Introduction: Olfactory dysfunction can be an early sign of Alzheimer's disease (AD). We used tau positron emission tomography-magnetic resonance (PET-MR) to analyze a key region of the olfactory circuit, the piriform cortex, in comparison to the adjacent medial temporal lobe.
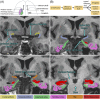
Methods: Using co-registered magnetic resonance imaging (MRI) and 18F-PI-2620 tau PET-MR scans in 94 older adults, we computed tau uptake in the piriform-periamygdaloid cortex, amygdala, entorhinal-perirhinal cortices, and hippocampus.
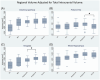
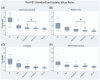
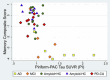
Results: We found an ordinal cross-sectional increase in piriform cortex tau uptake with increasing disease severity (amyloid-negative controls, amyloid-positive controls, mild cognitive impairment [MCI] and AD), comparable to entorhinal-perirhinal cortex. Amyloid-positive controls showed significantly greater tau uptake than amyloid-negative controls. Negative correlations were present between memory performance and piriform uptake. Piriform uptake was not elevated in cognitively unimpaired Parkinson's disease.
Discussion: Cross-sectionally, there is an early increase in tau uptake in the piriform cortex in AD but not in Parkinson's disease.
Highlights: Positron emission tomography-magnetic resonance (PET-MR) analysis of the piriform cortex sheds light on its role as a potential early region affected by neurodegenerative disorders underlying olfactory dysfunction.Uptake of tau tracer was elevated in the piriform cortex in Alzheimer's disease (AD) and mild cognitive impairment (MCI) but not in Parkinson's disease (PD).Memory performance was worse with greater piriform uptake.
Keywords: Alzheimer's; MRI; PD; PET; PET‐MR; olfaction; piriform cortex; tau.
© 2024 The Author(s). Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Dr Kathleen Poston has been funded by grants to conduct research from the Michael J Fox Foundation for Parkinson's Research, the Knight Initiative for Brain Resilience, the Wu Tsai Neurosciences Institute, the Lewy Body Dementia Association, the Alzheimer's Drug Discovery Foundation, the Sue Berghoff LBD Research Fellowship, and the National Institutes of Health (NIH). She is on the Scientific Advisory Board for Curasen where she receives consulting fees and stock options. She is on the Scientific Advisory Board for Amprion, where she receives stock options. She is a consultant for Novartis, Biohaven, and Neuron23, where she receives consulting fees. Dr Michael Zeineh receives research funding from GE Healthcare. All other authors have no disclosures relevant to this manuscript. Author disclosures are available in the Supporting Information.
Figures
References
-
- Doty RL. Handbook of Olfaction and Gustation. 3rd ed. 2015:1‐1264. doi: 10.1002/9781118971758. Published online June. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
