Mechanistic insights and therapeutic potential of astilbin and apigenin in diabetic cardiomyopathy
- PMID: 39583813
- PMCID: PMC11582444
- DOI: 10.1016/j.heliyon.2024.e39996
Mechanistic insights and therapeutic potential of astilbin and apigenin in diabetic cardiomyopathy
Abstract
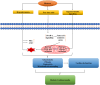
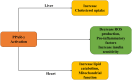
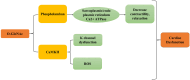
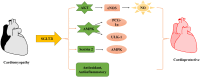
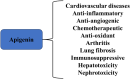
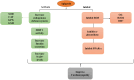
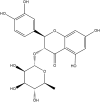
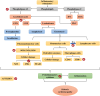
Diabetic cardiomyopathy (DCM) represents a critical complication of Diabetes mellitus (DM), characterized by structural and functional changes in the myocardium independent of coronary artery disease or hypertension. Emerging evidence highlights the significant roles of phytochemicals, particularly astilbin and apigenin, in modulating key molecular pathways implicated in DCM. This review synthesizes current mechanistic insights and therapeutic potential of these compounds, focusing on their interactions with AMP-activated protein kinase (AMPK), peroxisome proliferator-activated receptors (PPARs), O-linked N-acetylglucosamine (O-GlcNAc), sodium-glucose co-transporter 2 (SGLT2), protein kinase C (PKC), nuclear factor kappa B (NF-κB), mitogen-activated protein kinase (MAPK), and c-Jun N-terminal kinase (JNK) pathways. Astilbin and apigenin have demonstrated the ability to improve cardiac function, mitigate oxidative stress, and reduce inflammatory responses in diabetic conditions. By activating AMPK and PPARs, these flavonoids enhance glucose uptake and fatty acid oxidation, contributing to improved metabolic homeostasis. Their inhibition of O-GlcNAcylation, SGLT2 activity, and PKC signaling further attenuates hyperglycemia-induced cellular damage. Additionally, suppression of NF-κB, MAPK, and JNK pathways by astilbin and apigenin results in reduced pro-inflammatory cytokine production and apoptotic cell death. Collectively, these interactions position astilbin and apigenin as promising therapeutic agents for ameliorating DCM, offering novel avenues for treatment strategies aimed at modulating multiple pathogenic pathways.
Keywords: Apigenin; Astilbin; Cardiomyopathy; Diabetes.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Whelton P.K., Williams B. The 2018 European society of cardiology/European society of hypertension and 2017 American college of cardiology/American heart association blood pressure guidelines: more similar than different. JAMA. 2018;320(17):1749–1750. - PubMed
-
- Davies M.J., et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American diabetes association (ada) and the European association for the study of diabetes (easd) Diabetologia. 2018;61:2461–2498. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

