Elevations of Cardiac Troponin in Patients Receiving Immune Checkpoint Inhibitors: Data From a Prospective Study
- PMID: 39583866
- PMCID: PMC11584941
- DOI: 10.1016/j.jacadv.2024.101375
Elevations of Cardiac Troponin in Patients Receiving Immune Checkpoint Inhibitors: Data From a Prospective Study
Abstract
Background: Immune checkpoint inhibitors (ICIs) are increasingly used in the treatment of cancer. However, immune-related adverse events are prevalent in patients receiving ICI therapy. A serious immune-related adverse event is ICI-myocarditis, which is complex to diagnose given that the significance of early symptoms and biomarker trajectories, such as high-sensitivity troponin T (hs-TnT) are unclear.
Objectives: The purpose of the study was to evaluate kinetics of hs-TnT in cancer patients receiving ICI and to identify patients at risk of developing ICI-myocarditis.
Methods: This prospective, observational, single-center study included 164 patients receiving ICI therapy. Patients' history, demographics, and clinical characteristics, as well as survival statistics, were collected from electronic patient records and used to analyze associations between elevated hs-TnT (≥14 ng/L) and a significant rise in hs-TnT (100% rise from baseline, with an absolute value ≥2x upper limit of normal (ie, ≥28 ng/L) with ICI-myocarditis.
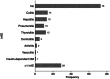
Results: We included 164 patients with a mean follow-up time of 1.60 ± 0.91 years. Melanoma was the most common type of cancer in the patient population, and most patients received treatment with programmed cell death protein 1 (PD-1). Twenty-six patients (16%) exhibited significant hs-TnT elevations, while 8 patients (5%) developed ICI-myocarditis. In 18 of 26 (69%) patients, ICI-myocarditis could not be diagnosed with certainty, while 10 of 26 (38%) patients had no other signs of symptoms of cardiac damage. All 8 myocarditis cases were preceded by significantly higher hs-TnT elevations than asymptomatic patients. Despite a high ICI-myocarditis incidence in our study population, cardiac mortality remained low (4%).
Conclusions: Significant hs-TnT elevations occur more often than previously reported, are often asymptomatic, and do not always lead to myocarditis diagnosis.
Keywords: ICI; cardiac biomarkers; hs-TnT; hypertroponinemia; immunotherapy; myocarditis.
© 2024 The Authors.
Conflict of interest statement
This work was supported by a grant from the 10.13039/501100000781European Research Council (ERC CoG 818715, SECRETE-HF). Support was received from grants from the Netherlands Heart Foundation (CVON SHE-PREDICTS-HF, grant2017-21; CVON RED-CVD, grant 2017-11; CVON PREDICT2, grant 2018-30; and CVON DOUBLE DOSE, grant 2020B005) and by a grant from the 10.13039/501100001674LeDucq Foundation (Cure PhosphoLambaN induced Cardiomyopathy [Cure-PLaN]). Dr Meijers is supported by the Mandema-Stipendium of the Junior Scientific Masterclass 2020-10 of the 10.13039/501100005075University Medical Center Groningen and by the Dutch Heart Foundation (Dekker grant 03-005-2021-T005). The UMCG, which employs/employed several of the authors, has received research grants and/or fees from AstraZeneca, Abbott, Boehringer Ingelheim, Cardior Pharmaceuticals GmbH, Novo Nordisk, and Roche; Dr de Boer has had speaker engagements with and/or received fees from and/or served on an advisory board for Abbott, AstraZeneca, Bristol Myers Squibb, Cardior Pharmaceuticals GmbH, NovoNordisk, and Roche; and received travel support from Abbott, Cardior Cardior Pharmaceuticals GmbH, and NovoNordisk. Dr van der Meer has received grant support or consultancy fees from Novartis, Pharma Nord, Pfizer, Ionis, Astra Zeneca, Vifor Pharma, Pharmacosmos, BridgeBio, and NovoNordisk. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures
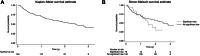

References
-
- Bagchi S., Yuan R., Engleman E.G. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–249. - PubMed
-
- Martins F., Sofiya L., Sykiotis G.P., et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563–580. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

