Prostaglandin E2 production in the brainstem parabrachial nucleus facilitates the febrile response
- PMID: 39583895
- PMCID: PMC11583619
- DOI: 10.1080/23328940.2024.2401674
Prostaglandin E2 production in the brainstem parabrachial nucleus facilitates the febrile response
Abstract
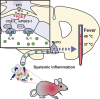
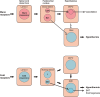
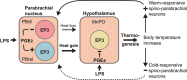
Our body temperature is normally kept within a narrow range of 1°C. For example, if our body temperature rises, such as in a hot environment or due to strenuous exercise, our thermoregulatory system will trigger a powerful heat defense response with vasodilation, sweating, and lowered metabolism. During fever, which often involves body temperatures of up to 41°C, this heat defense mechanism is apparently inhibited; otherwise, the rising body temperature would be immediately combated, and fever would not be allowed to develop. New evidence suggests how and where this inhibition takes place. In two consecutive studies from Cheng et al. and Xu et al., it has been shown that prostaglandin E2, which generates fever by acting on thermosensory neurons in the preoptic hypothalamus, also acts on neurons in the brainstem parabrachial nucleus, which receive temperature information from temperature-activated spinal cord neurons and relay this information to the thermoregulatory center in the hypothalamus to either induce cold or heat defenses. By acting on the same type of prostaglandin E2 receptor that is critical for fever generation in the preoptic hypothalamus, the EP3 receptor, prostaglandin E2 inhibits the signaling of the heat-responsive parabrachial neurons, while stimulating the cold-responsive neurons. These novel findings thus show that prostaglandin E2, by binding to the same receptor subtype in the parabrachial nucleus as in the preoptic hypothalamus, adjusts the sensitivity of the thermosensory system in a coordinated manner to allow the development of febrile body temperatures.
Keywords: EP3 receptors; Fever; PGE2; median preoptic nucleus; thermosensory pathways.
© 2024 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
