Phase II trial of dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors SWOG/NCI experience: invasive mucinous or non-mucinous lepidic adenocarcinoma of the lung (formerly bronchioloalveolar carcinoma)
- PMID: 39583952
- PMCID: PMC11583498
- DOI: 10.1177/17588359241293401
Phase II trial of dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors SWOG/NCI experience: invasive mucinous or non-mucinous lepidic adenocarcinoma of the lung (formerly bronchioloalveolar carcinoma)
Abstract
Background: Anti-programmed death-1 (PD-1)/cytotoxic T lymphocyte antigen-4 antibodies are efficacious in various malignancies.
Objectives: This study presents the first results of ipilimumab-nivolumab in invasive mucinous or non-mucinous lepidic adenocarcinoma (invasive mucinous adenocarcinoma (IMA) or invasive non-mucinous lepidic adenocarcinomas (INLA), respectively) of the lung.
Design: Dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors (DART) is a prospective, open-label, multicenter (1016 US sites), multi-cohort phase II trial of ipilimumab (1 mg/kg intravenously (IV) every 6 weeks) plus nivolumab (240 mg IV every 2 weeks).
Methods: Participants histologically diagnosed with advanced IMA or INLA, who had not responded to at least one line of therapy, were included in the bronchioloalveolar carcinoma cohort. The primary endpoint was the overall response rate (ORR) by Response Evaluation Criteria in Solid Tumors (confirmed complete and partial responses (CR and PR)). Secondary endpoints were progression-free survival (PFS), overall survival (OS), clinical benefit rate (CBR; stable disease (SD) ⩾ 6 months plus ORR), and toxicity.
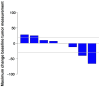
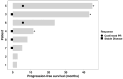
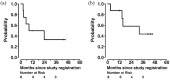
Results: Eight evaluable patients (median age: 77 years; the number of prior therapies ranged from 0 to 4; one patient with prior exposure to a PD-1 inhibitor; comprising six IMA and two INLA) were treated. One IMA had a 40% regression (PFS 45.2+ months, PD-L1 0%, KRAS G12C mutated, tumor mutational burden [TMB] 13 mut/Mb). One INLA had 66% regression (PFS 23.8 months, PD-L1 unknown, no actionable mutations, TMB 3 mut/Mb). Overall ORR was 25.0% (2/8) and CBR, 62.5% (5/8); PFS for the patients with SD > 6 months was 43.4+, 11.7+, and 8.3 months. The median PFS was 16 months (5.3-not reached) and the median OS was 32.2 months (14.6-not reached). The toxicity profile was similar to previous reports.
Conclusion: Ipilimumab plus nivolumab in the bronchioloalveolar carcinoma cohort (IMA, INLA) resulted in a durable ORR of 25.0% and CBR of 62.5% (PFS, 8.3 11.7+. 23.8 (PR), 43.4+ and 45.2+ (PR) months). Correlative studies to determine response and resistance markers are ongoing. Expanded prospective studies are warranted.
Trial registration: ClinicalTrials.gov registry: NCT02834013.
Keywords: DART; invasive mucinous adenocarcinoma; ipilimumab; lepidic adenocarcinoma; nivolumab.
© The Author(s), 2024.
Conflict of interest statement
Dr Y.K.C. reports research grants from AbbVie, Bristol-Myers Squibb, Biodesix, Freenome, and Predicine, and consulting fees, payments, and/or honoraria from Roche/Genentech, AstraZeneca, Foundation Medicine, Neogenomics, Guardant Health, Boehringer Ingelheim, Biodesix, ImmuneOncia, Lilly Oncology, Merck, Takeda, Lunit, Jazz Pharmaceutical, Tempus, Bristol-Myers Squibb, Regeneron, NeoImmunTech, and Esai. Dr M.O. reports consulting fees from Merck and Biosight. She serves on the Data Safety Monitoring Boards for Bristol Myers Squibb (BMS), Glycomimetics, and Grifols. Dr C.W.R. reports research funding from Ayala, Deciphera, Daiichi-Sankyo, Karyopharm, PTC Therapeutics, Shasqi, PF Argentum IP Holdings, LLC, RainTherapeutics, BMS, Exelixis, Genentech, Novartis, Merck, Nektar, Pfizer, and Nikang Therapeutics; as well as expert testimony for Pfizer, GSK, and Boehringer-Ingelheim. Dr D.E.G. reports research funding from Astra-Zeneca, BerGenBio, Karyopharm, and Novocure; consulting fees from Catalyst Pharmaceuticals; advisory board participation for Astra-Zeneca, Daiichi-Sankyo, Elevation Oncology, Janssen Scientific Affairs, Jazz Pharmaceuticals, Regeneron Pharmaceuticals, and Sanofi; stock holdings in Gilead; U.S. patent 11,747,345; pending patents 17/045,482, 63/386,387, 63/382,972, 63/382,257; and is co-founder of OncoSeer Diagnostics, LLC. Dr R.K. has received research funding from Boehringer Ingelheim, Debiopharm, Foundation Medicine, Genentech, Grifols, Guardant, Incyte, Konica Minolta, MedImmune, Merck Serono, Omniseq, Pfizer, Sequenom, Takeda, and TopAlliance and from the NCI; as well as consultant and/or speaker fees and/or advisory board/consultant for Actuate Therapeutics, AstraZeneca, Bicara Therapeutics, Inc., Biological Dynamics, Caris, Datar Cancer Genetics, Daiichi, EISAI, EOM Pharmaceuticals, Iylon, LabCorp, Merck, NeoGenomics, Neomed, Pfizer, Prosperdtx, Regeneron, Roche, TD2/Volastra, Turning Point Therapeutics, X-Biotech; has an equity interest in CureMatch Inc. and IDbyDNA; serves on the Board of CureMatch and CureMetrix, and is a co-founder of CureMatch.
Figures
References
-
- Read WL, Page NC, Tierney RM, et al. The epidemiology of bronchioloalveolar carcinoma over the past two decades: analysis of the SEER database. Lung Cancer 2004; 45: 137–142. - PubMed
-
- Travis WD, Garg K, Franklin WA, et al. Evolving concepts in the pathology and computed tomography imaging of lung adenocarcinoma and bronchioloalveolar carcinoma. J Clin Oncol 2005; 23: 3279–3287. - PubMed
-
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015; 10: 1243–1260. - PubMed
-
- Nicholson AG, Tsao MS, Beasley MB, et al. The 2021 WHO Classification of Lung Tumors: impact of advances since 2015. J Thorac Oncol 2022; 17: 362–387. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

