Investigation of the atomic layer etching mechanism for Al2O3 using hexafluoroacetylacetone and H2 plasma
- PMID: 39583983
- PMCID: PMC11582961
- DOI: 10.1039/d4tc03615h
Investigation of the atomic layer etching mechanism for Al2O3 using hexafluoroacetylacetone and H2 plasma
Abstract
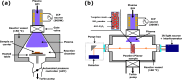
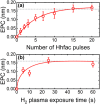
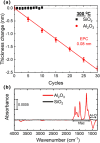
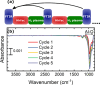
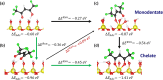
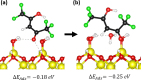
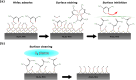
Atomic layer etching (ALE) is required to fabricate the complex 3D structures for future integrated circuit scaling. To enable ALE for a wide range of materials, it is important to discover new processes and subsequently understand the underlying mechanisms. This work focuses on an isotropic plasma ALE process based on hexafluoroacetylacetone (Hhfac) doses followed by H2 plasma exposure. The ALE process enables accurate control of Al2O3 film thickness with an etch rate of 0.16 ± 0.02 nm per cycle, and an ALE synergy of 98%. The ALE mechanism is investigated using Fourier transform infrared spectroscopy (FTIR) and density functional theory (DFT) simulations. Different diketone surface bonding configurations are identified on the Al2O3 surface, suggesting that there is competition between etching and surface inhibition reactions. During the Hhfac dosing, the surface is etched before becoming saturated with monodentate and other hfac species, which forms an etch inhibition layer. H2 plasma is subsequently employed to remove the hfac species, cleaning the surface for the next half-cycle. On planar samples no residue of the Hhfac etchant is observed by FTIR after H2 plasma exposure. DFT analysis indicates that the chelate configuration of the diketone molecule is the most favorable surface species, which is expected to leave the surface as volatile etch product. However, formation of the other configurations is also energetically favorable, which explains the buildup on an etch inhibiting layer. The ALE process is thus hypothesized to work via an etch inhibition and surface cleaning mechanism. It is discussed that such a mechanism enables thickness control on the sub-nm scale, with minimal contamination and low damage.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
-
- Kanarik K. J. Lill T. Hudson E. A. Sriraman S. Tan S. Marks J. Vahedi V. Gottscho R. A. J. Vac. Sci. Technol., A. 2015;33:020802. doi: 10.1116/1.4913379. - DOI
-
- Fischer A., Janek R., Boniface J., Lill T., Kanarik K. J., Pan Y., Vahedi V. and Gottscho R. A., in Advanced Etch Technology for Nanopatterning VI, ed. S. U. Engelmann and R. S. Wise, American Vacuum Society, 2017, vol. 10149, p. 101490H
-
- Carver C. T. Plombon J. J. Romero P. E. Suri S. Tronic T. A. Turkot R. B. ECS J. Solid State Sci. Technol. 2015;4:N5005–N5009. doi: 10.1149/2.0021506jss. - DOI
-
- Clark R. Tapily K. Yu K.-H. Hakamata T. Consiglio S. O’Meara D. Wajda C. Smith J. Leusink G. APL Mater. 2018;6:058203. doi: 10.1063/1.5026805. - DOI
LinkOut - more resources
Full Text Sources
