Bridging immune-neurovascular crosstalk via the immunomodulatory microspheres for promoting neural repair
- PMID: 39584066
- PMCID: PMC11583666
- DOI: 10.1016/j.bioactmat.2024.10.031
Bridging immune-neurovascular crosstalk via the immunomodulatory microspheres for promoting neural repair
Abstract
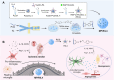
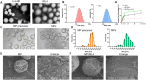
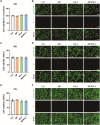
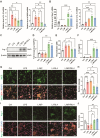
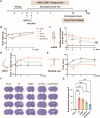
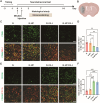
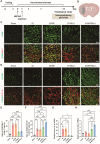
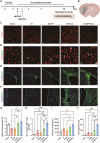
The crosstalk between immune cells and the neurovascular unit plays a pivotal role in neural regeneration following central nervous system (CNS) injury. Maintaining brain immune homeostasis is crucial for restoring neurovascular function. In this study, an interactive bridge was developed via an immunomodulatory hydrogel microsphere to link the interaction network between microglia and the neurovascular unit, thereby precisely regulating immune-neurovascular crosstalk and achieving neural function recovery. This immunomodulatory crosstalk microsphere (MP/RIL4) was composed of microglia-targeted RAP12 peptide-modified interleukin-4 (IL-4) nanoparticles and boronic ester-functionalized hydrogel using biotin-avidin reaction and air-microfluidic techniques. We confirmed that the immunomodulatory microspheres reduced the expression of pro-inflammatory factors including IL-1β, iNOS, and CD86, while upregulating levels of anti-inflammatory factors such as IL-10, Arg-1, and CD206 in microglia. In addition, injection of the MP/RIL4 significantly mitigated brain atrophy volume in a mouse model of ischemic stroke, promoted neurobehavioral recovery, and enhanced the crosstalk between immune cells and the neurovascular unit, thus increasing angiogenesis and neurogenesis of stroke mice. In summary, the immunomodulatory microspheres, capable of orchestrating the interaction between immune cells and neurovascular unit, hold considerable therapeutic potential for ischemic stroke and other CNS diseases.
Keywords: Angiogenesis; Crosstalk; Immune modulating microsphere; Ischemic stroke; Neurogenesis.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Baldino L., et al. Regeneration techniques for bone-to-tendon and muscle-to-tendon interfaces reconstruction. Br. Med. Bull. 2016;117(1):25–37. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

