Development and characterization of high-efficiency cell-adapted live attenuated vaccine candidate against African swine fever
- PMID: 39584308
- PMCID: PMC11610309
- DOI: 10.1080/22221751.2024.2432372
Development and characterization of high-efficiency cell-adapted live attenuated vaccine candidate against African swine fever
Abstract
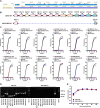
African swine fever (ASF), a contagious and lethal haemorrhagic disease of domestic pigs and wild boars, poses a significant threat to the global pig industry. Although experimental vaccine candidates derived from naturally attenuated, genetically engineered, or cell culture-adapted ASF virus have been tested, no commercial vaccine is accepted globally. We developed a safe and effective cell-adapted live attenuated vaccine candidate (ASFV-MEC-01) by serial passage of a field isolate in CA-CAS-01-A cells. ASFV-MEC-01, isolated via repeated plaque purification using next-generation sequencing analysis, was obtained at passage 18 and showed significant attenuation in 4- and 6-week-old pigs. ASFV-MEC-01 conferred 100% protection against challenge with lethal parental ASFV, which correlated with high ASFV-specific humoral and cellular immune responses. Additionally, ASFV-MEC-01 was not detected in blood until 28 days post-inoculation. Global transcriptome analysis showed that ASFV-MEC-01 lacking 12 genes triggered stronger innate antiviral responses than the parental virus, as exemplified by high levels of mRNA encoding interferon regulatory and inflammatory genes in PAM cells. Ectopic expression of most deleted genes increased replication of DNA viruses by suppressing production of interferons and pro-inflammatory cytokines. Among the genes deleted from ASFV-MEC-01, MGF100-1R interacted specifically with the scaffold dimerization domain of TBK1, thereby preventing TBK1 dimerization and impairing TBK1-mediated type I IFN and NF-κB signalling. These results suggest that attenuation of ASFV-MEC-01 may be mediated by induction of stronger type I IFN and NF-κB signalling within the host innate immune system. Thus, ASFV-MEC-01 could be a safe and effective live attenuated ASFV vaccine candidate with commercial potential.
Keywords: African swine fever, CA-CAS-01-A cell, live-attenuated virus, ASFV-MEC-01, vaccine, Type I IFN, MGF100-1R.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
-
- Ranganatha S, Rathnamma D, Isloor S, et al. . African swine fever: analysing its epidemiology, pathogenesis and control strategies: A review. Indian J Anim Res; 1(10.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
