Overexpression of enhanced yellow fluorescent protein fused with Channelrhodopsin-2 causes contractile dysfunction in skeletal muscle
- PMID: 39584396
- PMCID: PMC11586894
- DOI: 10.1096/fj.202401664RR
Overexpression of enhanced yellow fluorescent protein fused with Channelrhodopsin-2 causes contractile dysfunction in skeletal muscle
Abstract
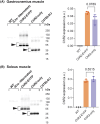
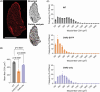
Skeletal muscle activation using optogenetics has emerged as a promising technique for inducing noninvasive muscle contraction and assessing muscle function both in vivo and in vitro. Transgenic mice overexpressing the optogenetic fusion protein, Channelrhodopsin 2-EYFP (ChR2-EYFP) in skeletal muscle are widely used; however, overexpression of fluorescent proteins can negatively impact the functionality of activable tissues. In this study, we characterized the contractile properties of ChR2-EYFP skeletal muscle and introduced the ChR2-only mouse model that expresses light-responsive ChR2 without the fluorescent EYFP in their skeletal muscles. We found a significant reduction in the contractile ability of ChR2-EYFP muscles compared with ChR2-only and WT mice, observed under both electrical and optogenetic stimulation paradigms. Bulk RNAseq identified the downregulation of genes associated with transmembrane transport and metabolism in ChR2-EYFP muscle, while the ChR2-only muscle did not demonstrate any notable deviations from WT muscle. The RNAseq results were further corroborated by a reduced protein-level expression of ion channel-related HCN2 in ChR2-EYFP muscles and gluconeogenesis-modulating FBP2 in both ChR2-EYFP and ChR2-only muscles. Overall, this study reveals an intrinsic skeletal dysfunction in the widely used ChR2-EYFP mice model and underscores the importance of considering alternative optogenetic models, such as the ChR2-only, for future research in skeletal muscle optogenetics.
Keywords: Channelrhodopsin‐2; function; optogenetics; skeletal muscle; structure.
© 2024 The Author(s). The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Figures






References
-
- Peckham PH, Knutson JS. Functional electrical stimulation for neuromuscular applications. Annu Rev Biomed Eng. 2005;7:327‐360. - PubMed
-
- Thomas CK, Zaidner EY, Calancie B, Broton JG, Bigland‐Ritchie BR. Muscle weakness, paralysis, and atrophy after human cervical spinal cord injury. Exp Neurol. 1997;148:414‐423. - PubMed
MeSH terms
Substances
Grants and funding
- K12 HD073945/HD/NICHD NIH HHS/United States
- K12HD073945/HHS | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
- R03 HD094594/HD/NICHD NIH HHS/United States
- ZIA HL006221/ImNIH/Intramural NIH HHS/United States
- 1ZIAHL006221/HHS | NIH | NHLBI | Division of Intramural Research (DIR)
- P30 AR069620/AR/NIAMS NIH HHS/United States
- P30AR069620/HHS | NIH | National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)
- 1944448/National Science Foundation (NSF)
- R01AR079367/HHS | NIH | National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)
- R03HD094594/HHS | NIH | National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)
- R01 AR079367/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

