A systematic analysis of read-across adaptations in testing proposal evaluations by the European Chemicals Agency
- PMID: 39584503
- PMCID: PMC11976166
- DOI: 10.14573/altex.2408292
A systematic analysis of read-across adaptations in testing proposal evaluations by the European Chemicals Agency
Abstract
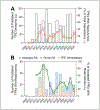
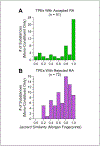
An essential aspect of the EU’s Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation is the European Chemicals Agency’s (ECHA) evaluation of testing proposals submitted by registrants to address data gaps. Registrants may propose adaptations, such as read-across, to waive standard testing; however, it is widely believed that ECHA often finds justifications for read-across hypotheses inadequate. From 2008 to August 2023, 2,630 testing proposals were submitted to ECHA; of these, 1,538 had published decisions that were systematically evaluated in this study. Each document was manually reviewed and information extracted for further analyses, focusing on 17 assessment elements (AEs) from the Read-Across Assessment Framework (RAAF) and testing proposal evaluations (TPE). Each submission was classified as to the AEs relied upon by the registrants and by ECHA. Data was analyzed for patterns and associations. Adaptations were included in 23% (350) of proposals, with analogue (168) and group (136) read-across being most common. Of the 304 read-across hypotheses, 49% were accepted, with group read-across showing significantly higher odds of acceptance. Data analysis examined factors such as tonnage band (Annex), test guidelines, hypothesis AEs, and structural similarities of target and source substances. While decisions were often context-specific, several significant associations influencing acceptance emerged. Overall, this analysis provides a comprehensive overview of 15 years of experience with testing proposal-specific read-across adaptations by both registrants and ECHA. These data will inform future submissions as they identify most critical AEs to increase the odds of read-across acceptance.
Keywords: ECHA; OECD test guideline studies; REACH; adaptations; read-across.
Plain language summary
The European Union’s law requires companies to provide safety data on chemicals they produce or import. To avoid unnecessary testing in animals, companies can propose alternatives like “read-across”, which uses data from similar substances. However, the European Chemicals Agency often rejects these proposals if the reasoning is not convincing. 1,538 decisions on testing proposals submitted between 2008 and 2023 were reviewed for this study. We analyzed the factors that influenced the agency’s decisions, focusing on 17 criteria. About 23% of proposals included read-across adaptations, with nearly half of these (49%) found acceptable. Group read-across proposals were more likely to succeed than analogue approaches. The study identified patterns and key factors, such as the similarity between substances and the type of tests proposed, that affected approval rates. This research offers valuable insights to help companies improve their proposals and increase the chances of approval for alternatives to animal testing.
Conflict of interest statement
Conflict of interest
N. Ball is an employee of the Dow Chemical Company, which submitted several registrations and testing proposals for evaluation by ECHA. Other authors declare no conflicts of interest.
Figures







Update of
-
A Systematic Analysis of Read-Across Adaptations in Testing Proposal Evaluations by the European Chemicals Agency.bioRxiv [Preprint]. 2024 Aug 30:2024.08.29.610278. doi: 10.1101/2024.08.29.610278. bioRxiv. 2024. Update in: ALTEX. 2025;42(1):22-38. doi: 10.14573/altex.2408292. PMID: 39257792 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

