French-Speaking Network of Pharmacogenetics (RNPGx) Recommendations for Clinical Use of Mavacamten
- PMID: 39584620
- PMCID: PMC11739748
- DOI: 10.1002/cpt.3502
French-Speaking Network of Pharmacogenetics (RNPGx) Recommendations for Clinical Use of Mavacamten
Abstract
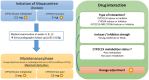
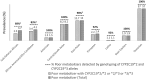
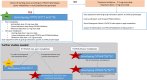
Mavacamten, the first drug in the class of β-cardiac myosin modulator, is used for the treatment of patients with hypertrophic cardiomyopathy. This orally administered drug demonstrates wide interpatient variability in pharmacokinetics parameters, due in part to variant CYP2C19 alleles. Individuals who are CYP2C19 poor metabolizers have increased exposure and are at increased risk of reduced cardiac hypercontractility. To ensure the safety of all patients, European Medicines Agency recommends CYP2C19 preemptive genotyping, and consecutively, to adapt maintenance and initial mavacamten doses, and to manage drug-drug interactions, according to CYP2C19 phenotype. In this article, we summarize evidence from the literature supporting the association between CYP2C19 phenotype and pharmacological features of mavacamten and provide, beyond biologic guidelines, therapeutic recommendations for the use of mavacamten based on CYP2C19 and CYP3A4/CYP3A5 genotype.
© 2024 The Author(s). Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work, and confirm that none of the authors participated in the clinical trials.
Figures
References
-
- Maron, B.J. , Gardin, J.M. , Flack, J.M. , Gidding, S.S. , Kurosaki, T.T. & Bild, D.E. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA study. Coronary artery risk development in (young) adults. Circulation 92, 785–789 (1995). - PubMed
-
- Maron, M.S. , Hellawell, J.L. , Lucove, J.C. , Farzaneh‐Far, R. & Olivotto, I. Occurrence of clinically diagnosed hypertrophic cardiomyopathy in the United States. Am. J. Cardiol. 117, 1651–1654 (2016). - PubMed
-
- Veselka, J. , Anavekar, N.S. & Charron, P. Hypertrophic obstructive cardiomyopathy. Lancet 389, 1253–1267 (2017). - PubMed
-
- Haute Autorité de Santé . Cardiomyopathie Hypertrophique (CMH) <https://www.has‐sante.fr/jcms/c_1100272/fr/cardiomyopathie‐hypertrophiqu...>. Accessed March 5, 2024.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

