Loss of the Endothelial Glycocalyx Component EMCN Leads to Glomerular Impairment
- PMID: 39584795
- PMCID: PMC11692785
- DOI: 10.1161/CIRCRESAHA.124.325218
Loss of the Endothelial Glycocalyx Component EMCN Leads to Glomerular Impairment
Abstract
Background: EMCN (endomucin), an endothelial-specific glycocalyx component, was found to be highly expressed by the endothelium of the renal glomerulus. We reported an anti-inflammatory role of EMCN and its involvement in the regulation of VEGF (vascular endothelial growth factor) activity through modulating VEGFR2 (VEGF receptor 2) endocytosis. The goal of this study is to investigate the phenotypic and functional effects of EMCN deficiency using the first global EMCN knockout mouse model.
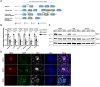
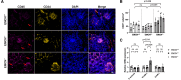
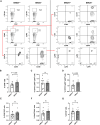
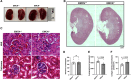
Methods: Global EMCN knockout mice were generated by crossing EMCN-floxed mice with ROSA26-Cre mice. Flow cytometry was used to analyze infiltrating myeloid cells in the kidneys. The ultrastructure of the glomerular filtration barrier was examined by transmission electron microscopy, whereas urinary albumin, creatinine, and total protein levels were analyzed from freshly collected urine samples. Expression and localization of EMCN, EGFP (enhanced green fluorescent protein), CD45 (cluster of differentiation 45), CD31, CD34, podocin, and albumin were examined by immunohistochemistry. Mice were weighed regularly, and their systemic blood pressure was measured using a noninvasive tail-cuff system. Glomerular endothelial cells and podocytes were isolated by fluorescence-activated cell sorting for RNA sequencing. Transcriptional profiles were analyzed to identify differentially expressed genes in both endothelium and podocytes, followed by gene ontology analysis. Protein levels of EMCN, albumin, and podocin were quantified by Western blot.
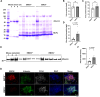
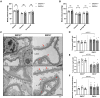
Results: The EMCN-/- mice exhibited increased infiltration of CD45+ cells, with an increased proportion of Ly6GhighLy6Chigh myeloid cells and higher VCAM-1 (vascular cell adhesion molecule 1) expression. EMCN-/- mice displayed albuminuria with increased albumin in the Bowman's space compared with the EMCN+/+ littermates. Glomeruli in EMCN-/- mice revealed fused and effaced podocyte foot processes and disorganized endothelial fenestrations. We found no significant difference in blood pressure between EMCN knockout mice and their wild-type littermates. RNA sequencing of glomerular endothelial cells revealed downregulation of cell-cell adhesion and MAPK (mitogen-activated protein kinase)/ERK (extracellular signal-regulated kinase) pathways, along with glycocalyx and extracellular matrix remodeling. In podocytes, we observed reduced VEGF signaling and alterations in cytoskeletal organization. Notably, there was a significant decrease in both mRNA and protein levels of podocin, a key component of the slit diaphragm.
Conclusion: Our study demonstrates a critical role of the endothelial marker EMCN in supporting normal glomerular filtration barrier structure and function by maintaining glomerular endothelial tight junction and homeostasis and podocyte function through endothelial-podocyte crosstalk.
Keywords: endothelium; glomerular filtration barrier; glycocalyx; inflammation; mice; podocytes.
Conflict of interest statement
None.
Figures


Comment in
-
Stick to the Script: How Endomucin Keeps the Glycocalyx in Line.Circ Res. 2025 Jan 3;136(1):75-77. doi: 10.1161/CIRCRESAHA.124.325805. Epub 2025 Jan 2. Circ Res. 2025. PMID: 39745991 Free PMC article. No abstract available.
References
-
- Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959 - PubMed
-
- Constantinescu AA, Vink H, Spaan JA. Endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. Arterioscler Thromb Vasc Biol. 2003;23:1541–1547. doi: 10.1161/01.ATV.0000085630.24353.3D - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

