Proprotein Convertase Subtilisin Kexin 9 Inhibitor in Severe Sepsis and Septic Shock Patients in a Phase II Prospective Cohort Study-Preliminary Results
- PMID: 39584843
- PMCID: PMC11586949
- DOI: 10.3390/idr16060083
Proprotein Convertase Subtilisin Kexin 9 Inhibitor in Severe Sepsis and Septic Shock Patients in a Phase II Prospective Cohort Study-Preliminary Results
Abstract
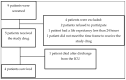
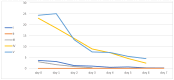
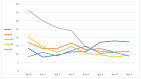
Sepsis is a life-threatening organ dysfunction syndrome caused by a dysregulated host response to infection that has a high mortality rate. Proprotein convertase subtilisin kexin 9 (PCSK9) is a serine protease secreted by the liver. Its binding to the low-density lipoprotein (LDL) receptor enhances its degradation, causing an increase in LDL levels in the blood. Objectives: Administering a PCSK9 inhibitor leading to an increase in lipid uptake by the liver may positively affect septic patients due to the increased removal of endotoxins. Methods: This preliminary study aimed to examine the safety of PCSK9 inhibitor use in septic and septic shock patients. We treated five septic patients in the intensive care unit with 300 mg of alirocumab following serious adverse events for 28 days. Results: Four of our patients did not experience any adverse events, and all of them survived. One patient died after discharge from the intensive care unit, and this death was presumably not related to the study drug. The patients rapidly recovered from the inflammatory stage of sepsis. Conclusions: Alirocumab appears safe in severe sepsis and septic shock patients. The outcome data are promising. Only a basic safety profile can be assessed based on this pilot study. Further study with a PCSK-9 inhibitor in septic or septic shock patients is required to further determine its benefit in ICU patients.
Keywords: PCSK9 inhibitor; alirocumab; sepsis; septic shock.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous