A Brief Chronicle of Antibody Research and Technological Advances
- PMID: 39584990
- PMCID: PMC11587137
- DOI: 10.3390/antib13040090
A Brief Chronicle of Antibody Research and Technological Advances
Abstract
This review briefly traces the historical development of antibody research and related technologies. The path from early perceptions of immunity to the emergence of modern immunotherapy has been marked by pivotal discoveries and technological advances. Early insights into immunity led to the development of vaccination and serotherapy. The elucidation of antibody structure and function paved the way for monoclonal antibody technology and its application in diagnosis and therapy. Breakthroughs in genetic engineering have enabled the production of humanized antibodies and the advances in Fc engineering, thereby increasing therapeutic efficacy. The discovery of immune checkpoints and cytokines revolutionized the treatment of cancer and autoimmune diseases. The field continues to evolve rapidly with the advent of antibody-drug conjugates, bispecific antibodies, and CAR T-cell therapies. As we face global health challenges, antibody research remains at the forefront of medical innovation and offers promising solutions for the future.
Keywords: antibody; immunoglobulin; immunology.
Conflict of interest statement
Author Ryota Maeda is employed by COGNANO Inc. and participated in the research as a co-author. Author Kazutaka Araki declares no commercial or financial relationships that could constitute potential conflicts of interest.
Figures

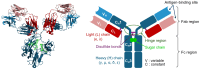





References
-
- Rees A.R. The Antibody Molecule: From Antitoxins to Therapeutic Antibodies. Oxford University Press; London, UK: 2014. Oxford Medical Histories.
-
- Marks L.V. The Lock and Key of Medicine: Monoclonal Antibodies and the Transformation of Healthcare. Yale University Press; New Haven, CT, USA: 2015.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

