Nuclear Focal Adhesion Kinase Protects against Cisplatin Stress in Ovarian Carcinoma
- PMID: 39585085
- PMCID: PMC11659947
- DOI: 10.1158/2767-9764.CRC-24-0382
Nuclear Focal Adhesion Kinase Protects against Cisplatin Stress in Ovarian Carcinoma
Abstract
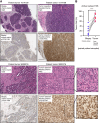
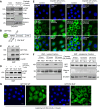
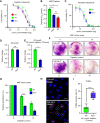
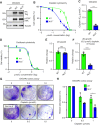
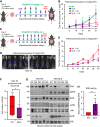
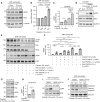
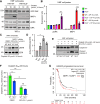
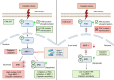
Abstract: Tumor chemotherapy resistance arises frequently and limits high-grade serous ovarian cancer (HGSOC) patient survival. Focal adhesion kinase (FAK) is an intracellular protein–tyrosine kinase encoded by PTK2, a gene that is often gained in HGSOC. Canonically, FAK functions at the cell periphery. However, FAK also transits to the nucleus to modulate gene expression. We find that FAK is tyrosine-phosphorylated and nuclear-localized in tumors of patients with HGSOC surviving neoadjuvant platinum–paclitaxel chemotherapy and that FAK nuclear accumulation occurs upon subcytotoxic cisplatin exposure to ovarian tumor cells in vitro. FAK nuclear localization sequence (NLS) mutational inactivation resulted in tumor cell sensitization to cisplatin in vitro and in vivo relative to wild-type FAK-reconstituted ovarian tumor cells. Cisplatin cytotoxicity was associated with elevated ERK MAPK activation in FAK NLS− cells, cisplatin-stimulated ERK activation was also enhanced upon loss of FAK activity or expression, and cisplatin-stimulated cell death was prevented by an inhibitor of ERK signaling. MAPK phosphastase-1 (MKP1) negatively regulates ERK signaling, and cisplatin-induced MKP1 levels were significantly elevated in wild-type FAK compared with FAK NLS− ovarian tumor cells. Notably, small-molecule MKP1 inhibition enhanced both cisplatin-stimulated ERK phosphorylation and ovarian tumor cell death. Together, our results show that FAK expression, activity, and nuclear localization limit cisplatin cytotoxicity in part by regulating MKP1 levels and preventing noncanonical ERK/MAPK activation.
Significance: FAK inhibitors are in combinatorial clinical testing with agents that prevent Ras-Raf-MAPK pathway activation in various cancers. This study suggests that nuclear FAK limits ERK/MAPK activation in supporting HGSOC cell survival to cisplatin stress. Overall, it is likely that targets of FAK-mediated survival signaling may be tumor type- and context-dependent.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
D.C. Connolly reports grants from the NCI during the conduct of the study. M.T. McHale reports grants from the UCSD during the conduct of the study. D.G. Stupack reports personal fees from Amplia Therapeutics outside the submitted work. No disclosures were reported by the other authors.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

