Lipid Nanoparticles With Fine-Tuned Composition Show Enhanced Colon Targeting as a Platform for mRNA Therapeutics
- PMID: 39585189
- PMCID: PMC11744673
- DOI: 10.1002/advs.202408744
Lipid Nanoparticles With Fine-Tuned Composition Show Enhanced Colon Targeting as a Platform for mRNA Therapeutics
Abstract
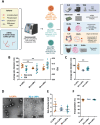
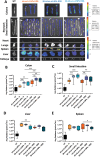
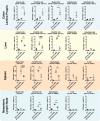
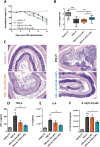
Lipid Nanoparticles (LNPs) recently emerged as an invaluable RNA delivery platform. With many LNP-based therapeutics in the pre-clinical and clinical pipelines, there is extensive research dedicated to improving LNPs. These efforts focus mainly on the tolerability and transfectability of new ionizable lipids and RNAs, or modulating LNPs biodistribution with active targeting strategies. However, most formulations follow the well-established lipid proportions used in clinically approved products. Nevertheless, investigating the effects of LNPs composition on their biodistribution can expand the toolbox for particle design, leading to improved delivery strategies. Herein, a new LNPs (30-n-LNPs) formulation with increasing amounts of phospholipids is investigated as a possible mRNA delivery system for treating Inflammatory Bowel Diseases. Compared to LNPs with benchmark composition (b-LNPs), n-LNPs containing 30% distearoylphosphatidylcholine (DSPC) are well tolerated following intravenous administration and display natural targeting toward the inflamed colon in dextran sodium sulfate (DSS)-colitis bearing mice, while de-targeting clearing organs such as the liver and spleen. Using interleukin-10-encoding mRNA as therapeutic cargo, n-LNPs demonstrated a reduction of pathological burden in colitis-bearing mice. n-LNPs represent a starting point to further investigate the influence of LNPs composition on systemic biodistribution, ultimately opening new therapeutic modalities in different pathologies.
Keywords: colon; genetic medicines; inflammatory bowel disease; lipid nanoparticles; mRNA; targeting.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
Listed in the manuscript under conflict of Interest (COI) only for Dan Peer. The rest of the authors have no COI.
Figures
References
-
- GBD 2017 Inflammatory Bowel Disease Collaborators , Lancet Gastroenterol. Hepatol. 2020, 5, 17. - PubMed
-
- de Souza H., Fiocchi C., Iliopoulos D., Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 739. - PubMed
-
- Roda G., Chien Ng S., Kotze P. G, Argollo M., Panaccione R., Spinelli A., Kaser A., Peyrin‐Biroulet L., Danese S., Nat. Rev. Dis. Primers 2020, 6, 22. - PubMed
-
- Kobayashi T., Siegmund B., Le Berre C., Wei S. C., Ferrante M., Shen B., Bernstein C. N., Danese S., Peyrin‐Biroulet L., Hibi T., Nat. Rev. Dis. Primers 2020, 6, 74. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
