SMOC1 colocalizes with Alzheimer's disease neuropathology and delays Aβ aggregation
- PMID: 39585417
- PMCID: PMC11588930
- DOI: 10.1007/s00401-024-02819-6
SMOC1 colocalizes with Alzheimer's disease neuropathology and delays Aβ aggregation
Abstract
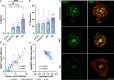
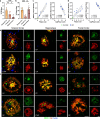
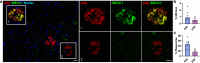
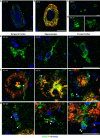
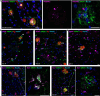
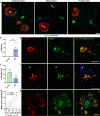
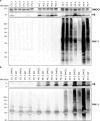
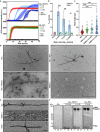
SMOC1 has emerged as one of the most significant and consistent new biomarkers of early Alzheimer's disease (AD). Recent studies show that SMOC1 is one of the earliest changing proteins in AD, with levels in the cerebrospinal fluid increasing many years before symptom onset. Despite this clear association with disease, little is known about the role of SMOC1 in AD or its function in the brain. Therefore, the aim of this study was to examine the distribution of SMOC1 in human AD brain tissue and to determine if SMOC1 influenced amyloid beta (Aβ) aggregation. The distribution of SMOC1 in human brain tissue was assessed in 3 brain regions (temporal cortex, hippocampus, and frontal cortex) using immunohistochemistry in a cohort of 73 cases encompassing advanced AD, mild cognitive impairment (MCI), preclinical AD, and cognitively normal controls. The Aβ- and phosphorylated tau-interaction with SMOC1 was assessed in control, MCI, and advanced AD human brain tissue using co-immunoprecipitation, and the influence of SMOC1 on Aβ aggregation kinetics was assessed using Thioflavin-T assays and electron microscopy. SMOC1 strongly colocalized with a subpopulation of amyloid plaques in AD (43.8 ± 2.4%), MCI (32.8 ± 5.4%), and preclinical AD (28.3 ± 6.4%). SMOC1 levels in the brain strongly correlated with plaque load, irrespective of disease stage. SMOC1 also colocalized with a subpopulation of phosphorylated tau aggregates in AD (9.6 ± 2.6%). Co-immunoprecipitation studies showed that SMOC1 strongly interacted with Aβ in human MCI and AD brain tissue and with phosphorylated tau in human AD brain tissue. Thioflavin-T aggregation assays showed that SMOC1 significantly delayed Aβ aggregation in a dose-dependent manner, and electron microscopy confirmed that the Aβ fibrils generated in the presence of SMOC1 had an altered morphology. Overall, our results emphasize the importance of SMOC1 in the onset and progression of AD and suggest that SMOC1 may influence pathology development in AD.
Keywords: Alzheimer’s disease; Beta amyloid; Electron microscopy; Immunohistochemistry; Mild cognitive impairment; Plaques; Preclinical; SMOC1; Tangles; Tau; Thioflavin T.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests.
Figures
Update of
-
SMOC1 colocalizes with Alzheimer's disease neuropathology and delays Aβ aggregation.Res Sq [Preprint]. 2024 Nov 1:rs.3.rs-5229472. doi: 10.21203/rs.3.rs-5229472/v1. Res Sq. 2024. Update in: Acta Neuropathol. 2024 Nov 25;148(1):72. doi: 10.1007/s00401-024-02819-6. PMID: 39574902 Free PMC article. Updated. Preprint.
References
-
- Afroz KF, Levites Y, Ryu D, Ran Y, Gadhavi JD, Liu X et al (2024) SPARC-related modular calcium-binding proteins (SMOC1 and 2) as a modulator of amyloid pathology in Alzheimer’s disease. Alzheimer’s association international conference
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

