Public Health Impact of Introducing a Pentavalent Vaccine Against Invasive Meningococcal Disease in the United States
- PMID: 39585581
- PMCID: PMC11825582
- DOI: 10.1007/s40273-024-01439-y
Public Health Impact of Introducing a Pentavalent Vaccine Against Invasive Meningococcal Disease in the United States
Abstract
Background: Invasive meningococcal disease (IMD) is primarily associated with five Neisseria meningitidis serogroups: A, B, C, W, or Y. In the United States (US), available vaccines protect against serogroups B (MenB), A, C, W, and Y (MenACWY), and A, B, C, W, and Y (MenABCWY). The Advisory Committee on Immunization Practices is re-evaluating the adolescent meningococcal vaccination schedule with varying recommendation formats. This analysis aimed to predict which schedule could avert the most IMD cases and have the most positive public health impact (PHI).
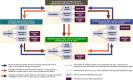
Methods: An epidemiological model compared the 15-year PHI of vaccination schedules using MenB, MenACWY, and/or MenABCWY vaccines versus current US standard of care (SoC). Varying coverage rates reflected routine, shared clinical decision making, and risk-based recommendations. Sensitivity analyses assessed robustness of the results to different inputs/assumptions.
Results: The most positive PHI compared with SoC was observed with one dose of MenACWY at 11 years of age and two doses of MenABCWY (6 months apart) at 16 years of age, assuming routine recommendation and coverage reflecting real-world uptake of MenACWY. This strategy resulted in 123 IMD cases averted (MenB: 59, MenACWY: 64), 17 deaths prevented, 574 life-years saved, and 757 quality-adjusted life-years gained versus SoC. Eliminating MenACWY vaccination at 11 years was found to result in an additional IMD burden.
Conclusion: A routinely recommended two-dose pentavalent vaccine, with doses administered 6 months apart at 16 years of age, alongside the routinely recommended MenACWY vaccine at 11 years of age, would improve the PHI and benefits of IMD vaccination to society.
© 2024. GlaxoSmithKline Biologicals.
Conflict of interest statement
Declarations. Funding: This study was sponsored by GSK. Support for third-party writing assistance for this article was funded by GSK in accordance with Good Publication Practice (GPP 2022) guidelines ( https://www.ismpp.org/gpp-2022 ). Conflict of Interest: HAS, OH-R, JC, DC, W-YS, EK, ZK: employee and shareholder of GSK; GJ: employee of and received consulting fees from GSK; JG, KAH, MG: employee of RTI Health Solutions, which received payment for this study from GSK; CB: employee and shareholder of GSK, shareholder of Pfizer; SB: employee of GSK. Data Availability Statement: The data generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Previous Presentations: Data obtained utilizing this model were presented at the European Society for Paediatric Infectious Diseases (ESPID) 2024 annual meeting. Authors’ Contributions: Substantial contributions to study conception and design: HAS, GJ, OH-R, ZK; substantial contributions to analysis or interpretation of the data: HAS, GJ, OH-R, JG, KAH, JC, MG, DC, CB, W-YS, EK, SB, ZK; drafting the article or revising it critically for important intellectual content: HAS, GJ, OH-R, JG, KAH, JC, MG, DC, CB, W-YS, EK, SB, ZK; final approval of the version of the article to be published: HAS, GJ, OH-R, JG, KAH, JC, MG, DC, CB, W-YS, EK, SB, ZK.
Figures

References
-
- Harrison LH, Pelton SI, Wilder-Smith A, Holst J, Safadi MA, Vazquez JA, et al. The Global Meningococcal Initiative: Recommendations for reducing the global burden of meningococcal disease. Vaccine. 2011;29(18):3363–71. - PubMed
-
- Pelton SI. The global evolution of meningococcal epidemiology following the introduction of meningococcal vaccines. J Adolesc Health. 2016;59(2 Suppl):S3–11. - PubMed
-
- Purmohamad A, Abasi E, Azimi T, Hosseini S, Safari H, Nasiri MJ, et al. Global estimate of Neisseria meningitidis serogroups proportion in invasive meningococcal disease: A systematic review and meta-analysis. Microb Pathog. 2019;134: 103571. - PubMed
-
- CDC. Revising the adolescent meningococcal vaccine schedule: Term of reference and considerations [online]. https://stacks.cdc.gov/view/cdc/148680. Accessed 12 Mar 2024.
-
- CDC. Meningococcal disease: Surveillance [online]. https://www.cdc.gov/meningococcal/php/surveillance/. Accessed 12 Mar 2024.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

