Neuropsychological Outcomes in 6-Year-Old Children of Women With Epilepsy: A Prospective Nonrandomized Clinical Trial
- PMID: 39585668
- PMCID: PMC11589855
- DOI: 10.1001/jamaneurol.2024.3982
Neuropsychological Outcomes in 6-Year-Old Children of Women With Epilepsy: A Prospective Nonrandomized Clinical Trial
Abstract
Importance: Antiseizure medications (ASMs) are potential teratogens commonly prescribed for multiple indications. ASM fetal exposure can impair neurodevelopment. Folate improves pregnancy outcomes, but higher doses may pose risks.
Objectives: To compare the outcomes of 6-year-old children of women with epilepsy (WWE) vs those of healthy women (HW), and assess the association of outcomes to third-trimester ASM exposures.
Design, setting, and participants: After informed consent, pregnant WWE and HW were enrolled from 2012 through 2016 in this prospective, multicenter, nonrandomized clinical trial. Children were assessed at 6 years of age (2019-2022). Participants were recruited from 20 US epilepsy centers. Study data were analyzed from August 2023 to August 2024.
Exposures: Fetal ASM exposures.
Main outcomes and measures: The a priori main neurodevelopmental outcome was the blindly assessed Verbal Index Score in 6-year-old children. The Verbal Index Score is calculated as the mean of the scores from the Word Definitions and Verbal Similarities subtests from the Differential Ability Scales, Expressive One-Word Picture Vocabulary Test, Phonological Processing, Comprehension of Instructions, and Sentence Repetition subtests from the Neuropsychological Assessment and Peabody Picture Vocabulary Test. The 2 primary analyses (1) compared children of WWE and HW using linear regression and (2) examined the outcomes of fetal exposure via ASM blood concentrations. Analyses were adjusted for multiple potential confounding factors. Other outcomes and folate exposure-related outcomes were assessed.
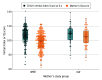
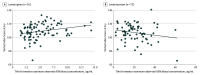
Results: A total of 1123 pregnant women were screened, and 456 were enrolled (426 did not meet criteria, and 241 chose not to participate). A total of 298 children of WWE (mean [SD] age, 6.4 [4.2] years; 158 female [53.0%]; 140 male [47.0%]) vs 89 children of HW (mean [SD] age, 6.4 [4.2] years; 41 female [46.1%]; 48 male [53.9%]) did not differ on Verbal Index Score (parameter estimate, -0.6; 95% CI, -3.2 to 1.9; P = .64). Exposure-dependent outcomes differed across ASMs. Assessment of other ASMs was limited because 232 of 298 WWE (78%) were taking lamotrigine or levetiracetam alone or in combination. Folate supplementation during the first 12 weeks of pregnancy had positive associations with cognition and behavior with no signal for risks at higher folate doses.
Conclusions and relevance: Results of this prospective nonrandomized clinical trial suggest that verbal abilities in children of WWE vs HW did not differ. Exposure-dependent outcomes of ASMs highlight the importance of dosing high enough to protect the mother and fetus from seizures but low enough to protect the fetus. Folate supplementation early in pregnancy including higher doses was associated with improved cognitive and behavioral outcomes. Additional research is needed for ASMs with inadequate information on fetal exposure risks.
Trial registration: ClinicalTrials.gov Identifier: NCT01730170.
Conflict of interest statement
Figures
References
-
- US Food and Drug Adminisration . FDA Drug safety communication: children born to mothers who took Valproate products while pregnant may have impaired cognitive development. Accessed June 20, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-c...

