Neisseria gonorrhoeae induces local secretion of IL-10 at the human cervix to promote colonization
- PMID: 39585777
- PMCID: PMC11735093
- DOI: 10.1172/JCI183331
Neisseria gonorrhoeae induces local secretion of IL-10 at the human cervix to promote colonization
Abstract
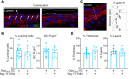
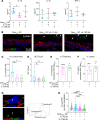
Gonorrhea, caused by the human-restricted pathogen Neisseria gonorrhoeae, is a commonly reported sexually transmitted infection. Since most infections in women are asymptomatic, the true number of infections is likely much higher than reported. How gonococci (GC) colonize women's cervixes without triggering symptoms remains elusive. Using a human cervical tissue explant model, we found that GC inoculation increased the local secretion of both proinflammatory (IL-1β and TNF-α) and antiinflammatory (IL-10) cytokines during the first 24 hours of infection. Cytokine induction required GC expression of Opa isoforms that bind the host receptors carcinoembryonic antigen-related cell adhesion molecules (CEACAMs). GC inoculation induced NF-κB activation in both cervical epithelial and subepithelial cells. However, inhibition of NF-κB activation, which reduced GC-induced IL-1β and TNF-α, did not affect GC colonization. Neutralizing IL-10 or blocking IL-10 receptors by antibodies reduced GC colonization by increasing epithelial shedding and epithelial cell-cell junction disassembly. Inhibition of the CEACAM downstream signaling molecule SHP1/2, which reduced GC colonization and increased epithelial shedding, decreased GC-induced IL-10 secretion. These results show that GC induce local secretion of IL-10, a potent antiinflammatory cytokine, at the cervix by engaging the host CEACAMs to prevent GC-colonizing epithelial cells from shedding, providing a potential mechanism for GC asymptomatic colonization in women.
Keywords: Bacterial infections; Cytokines; Infectious disease.
Conflict of interest statement
Figures



References
-
- CDC. Sexually transmitted infection surveillance 2022. https://www.cdc.gov/std/statistics/2022/default.htm#:~:text=Overall%2C%20in%202022%2C%20more%20than,in%20the%20past%20five%20years. Accessed November 14, 2024.

