AtFH5 recruits and transports the arabinogalactan protein AGP23 to maintain the tip growth of pollen tube
- PMID: 39585983
- PMCID: PMC11626185
- DOI: 10.1073/pnas.2410607121
AtFH5 recruits and transports the arabinogalactan protein AGP23 to maintain the tip growth of pollen tube
Abstract
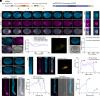
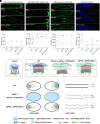
Actin cytoskeleton drives the targeted transport of cell wall components to sustain the tip growth of pollen tubes for double fertilization; however, the underlying mechanism remains largely unknown. Arabidopsis formin 5 (AtFH5), an actin-nucleating protein, localizes at secretory vesicles and mediates actin polymerization-based vesicle trafficking in pollen. Here, we demonstrate that AtFH5 determines the recruitment and transport of cell wall components in AtFH5-labeled vesicles during the tip growth of pollen tubes. Through a screen of interacting proteins of AtFH5, we identify many cell wall-related proteins, with arabinogalactan protein 23 (AGP23) occupying the highest frequency. AtFH5 interacts with AGP23 via its N-terminal extracellular domain (ECD) and jointly regulate the pollen germination and tube growth process. Further observations reveal that AGP23 co-localizes with AtFH5 at moving vesicles, germination sites, and pollen tube tips, suggesting that AGP23 is delivered by AtFH5-labeled vesicles. Deletion of the ECD of AtFH5 interrupts the dynamic localization and cell-wall connection of AGP23 in pollen grains and tubes. Cytological and genetic evidence shows that AGP23 and AtFH5 work in the same pathway to modulate cell wall composition. Together, our data uncover a role of formin in directing the sorting and deposition of cell wall components via secretory vesicle trafficking during pollen germination and tube growth.
Keywords: AGP23; AtFH5; cell wall; pollen; tip growth.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

