AgRP neurons mediate activity-dependent development of oxytocin connectivity and autonomic regulation
- PMID: 39585985
- PMCID: PMC11626166
- DOI: 10.1073/pnas.2403810121
AgRP neurons mediate activity-dependent development of oxytocin connectivity and autonomic regulation
Abstract
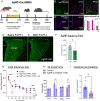
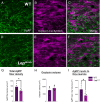
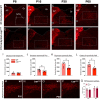
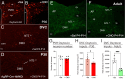
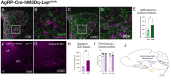
During postnatal life, leptin specifies neuronal inputs to the paraventricular nucleus of the hypothalamus (PVH) and activates agouti-related peptide (AgRP) neurons in the arcuate nucleus of the hypothalamus. Activity-dependent developmental mechanisms impact refinement of sensory circuits, but whether leptin-mediated postnatal neuronal activity specifies hypothalamic neural projections is largely unexplored. Here, we used chemogenetics to manipulate the activity of AgRP neurons during a discrete postnatal critical period and evaluated the development of AgRP inputs to the PVH and descending efferent outflow to the dorsal vagal complex (DVC). In leptin-deficient mice, targeting of AgRP neuronal outgrowth to PVH oxytocin neurons was reduced, and despite the lack of leptin receptors found on oxytocin neurons in the PVH, oxytocin-containing connections to the DVC were also impaired. Activation of AgRP neurons during early postnatal life not only normalized AgRP inputs to the PVH but also oxytocin outputs to the DVC in leptin-deficient mice. Blocking AgRP neuron activity during the same postnatal period reduced the density of AgRP inputs to the PVH of wild type mice, as well as the density of oxytocin-containing DVC fibers, and these innervation deficits were associated with dysregulated autonomic function. These findings suggest that leptin-mediated AgRP neuronal activity is required for the development of PVH connectivity and represents a unique activity-dependent mechanism for specification of neural pathways involved in the hypothalamic integration of autonomic responses.
Keywords: autonomic; hypothalamus; leptin; neural development; paraventricular hypothalamic nucleus.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

Update of
-
AgRP neurons mediate activity-dependent development of oxytocin connectivity and autonomic regulation.bioRxiv [Preprint]. 2024 Jun 3:2024.06.02.592838. doi: 10.1101/2024.06.02.592838. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2024 Dec 3;121(49):e2403810121. doi: 10.1073/pnas.2403810121. PMID: 38895484 Free PMC article. Updated. Preprint.
References
-
- Donato J., Programming of metabolism by adipokines during development. Nat. Rev. Endocrinol. 19, 385–397 (2023). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

