Podocyte SIRPα reduction in diabetic nephropathy aggravates podocyte injury by promoting pyruvate kinase M2 nuclear translocation
- PMID: 39586122
- PMCID: PMC11625355
- DOI: 10.1016/j.redox.2024.103439
Podocyte SIRPα reduction in diabetic nephropathy aggravates podocyte injury by promoting pyruvate kinase M2 nuclear translocation
Abstract
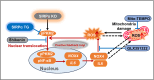
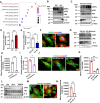
Podocyte injury is a critical event in the pathogenesis of diabetic nephropathy (DN). Hyperglycemia, oxidative stress, inflammation, and other factors contribute to podocyte damage in DN. In this study, we demonstrate that signaling regulatory protein alpha (SIRPα) plays a pivotal role in regulating the metabolic and immune homeostasis of podocytes. Deletion of SIRPα in podocytes exacerbates, while transgenic overexpression of SIRPα alleviates, podocyte injury in experimental DN mice. Mechanistically, SIRPα downregulation promotes pyruvate kinase M2 (PKM2) phosphorylation, initiating a positive feedback loop that involves PKM2 nuclear translocation, NF-κB activation, and oxidative stress, ultimately impairing aerobic glycolysis. Consistent with this mechanism, shikonin ameliorates podocyte injury by reducing PKM2 nuclear translocation, preventing oxidative stress and NF-κB activation, thereby restoring aerobic glycolysis.
Keywords: Aerobic glycolysis; NF-κB; Oxidative stress; PKM2; Podocyte injury; SIRPα.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Limin Li reports financial support was provided by the National Natural Science Foundation of China (32170897). Limin Li reports a relationship with the National Natural Science Foundation of China (32170897) that includes: funding grants. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

