NF-κB c-Rel is a critical regulator of TLR7-induced inflammation in psoriasis
- PMID: 39586195
- PMCID: PMC11625363
- DOI: 10.1016/j.ebiom.2024.105452
NF-κB c-Rel is a critical regulator of TLR7-induced inflammation in psoriasis
Abstract
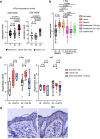
Background: Nuclear factor kappa B (NF-κB) c-Rel is a psoriasis susceptibility locus, however mechanisms underlying c-Rel transactivation during disease are poorly understood. Inflammation in psoriasis can be triggered following Toll-like Receptor 7 (TLR7) signalling in dendritic cells (DCs), and c-Rel is a critical regulator of DC function. Here, we studied the mechanism of TLR7-induced c-Rel-mediated inflammation in DCs.
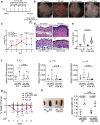
Methods: The overall expression of c-Rel was analysed in skin sections from patients with psoriasis in human transcriptomics datasets as well as the imiquimod-induced psoriasis mouse model. The function of c-Rel in DCs following TLR7 stimulation was determined by c-Rel CRISPR/Cas9 knockout DC2.4 immortalised cells and primary bone marrow derived dendritic cells from c-Rel knockout C57BL6/J mice.
Findings: c-Rel is highly expressed in lesional skin of patients with psoriasis and TLR7-induced psoriatic lesions in mice. c-Rel deficiency protected mice from the disease, and specifically compromised TLR7-induced, and not TLR9- or TLR3-induced, inflammation in dendritic cells. Mechanistically, c-Rel deficiency disrupted activating NF-κB dimers and allowed binding of inhibitory NF-κB homodimers to the IL-1β and IL-6 promoters thus inhibiting their expression. This functionally compromises the ability of c-Rel deficient DCs to induce Th17 polarisation, which is critical in psoriasis pathogenesis.
Interpretation: Our findings reveal that c-Rel is a key regulator of TLR7-mediated dendritic cell-dependent inflammation, and that targeting c-Rel-dependent signalling could prove an effective strategy to dampen excessive inflammation in TLR7-related skin inflammation.
Funding: A complete list of funding sources that contributed to this study can be found in the Acknowledgements section.
Keywords: Inflammation; NF-κB c-Rel; Psoriasis; TLR7; Transcription.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests KDC is Vice President-Elect of the American Academy of Dermatology and Treasurer of the International Eczema Council. The authors declare no further potential conflicts of interest.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

